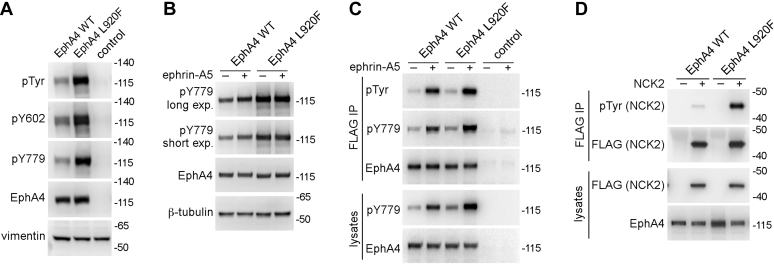
Figure 2.
The L920F mutation in the EphA4 SAM domain promotes receptor tyrosine phosphorylation and activation.A, HEK293 cells were transiently transfected with constructs encoding EphA4 WT, the EphA4 L920F mutant, or EGFP as a control. Cell lysates were probed by immunoblotting with antibodies to phosphotyrosine (pTyr), the Y602 and Y779 EphA4 phosphorylation sites, EphA4, and vimentin as a loading control. B, HEK293 cells were transiently transfected as indicated and treated for 10 min with 0.5 μg/ml ephrinA5-Fc (+) or Fc as a control (−). Cell lysates were probed by immunoblotting with antibodies to the Y779 EphA4 phosphorylation site (short and long exposures are shown), EphA4, and β-tubulin as a loading control. C, HEK293 cells were stably transfected with constructs encoding FLAG-tagged EphA4 WT or L920F mutant, or EGFP as a control, and treated for 10 min with 0.5 μg/ml ephrinA5-Fc (+) or Fc as a control (−). FLAG immmunoprecipitates and cell lysates were probed by immunoblotting with the indicated antibodies. D, HEK293 cells were transiently transfected with constructs encoding Strep-tagged EphA4 WT or L920F mutant with or without FLAG-tagged NCK2. FLAG immunoprecipitates and cell lysates were probed by immunoblotting with the indicated antibodies.