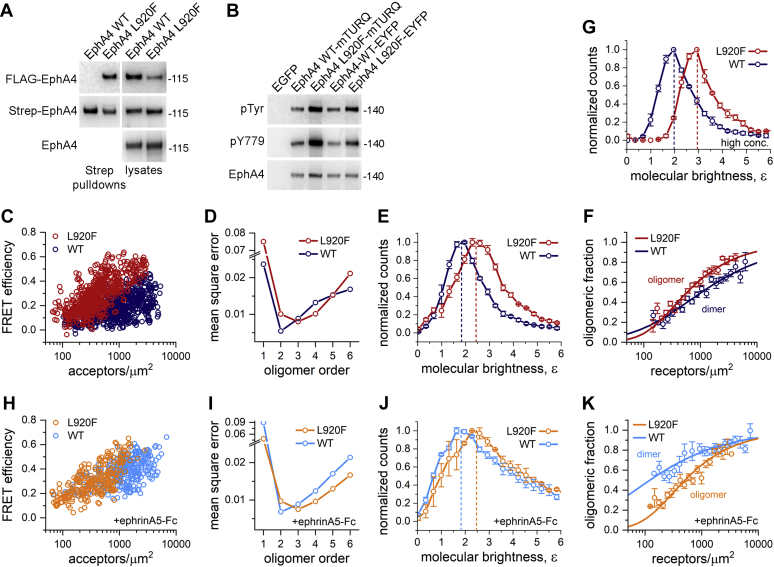
Figure 4.
The L920F mutation induces EphA4 oligomerization.A, HEK293 cells were transiently cotransfected with FLAG- or Strep-tagged EphA4 WT or L920F mutant. Cell lysates were subjected to pull-down with Strep-Tactin beads and the proteins bound to the beads were eluted, split into two aliquots and each aliquot was probed by immunoblotting with antibodies to the FLAG tag to detect FLAG-EphA4 or to the Strep tag to detect Strep-EphA4. Different aliquots of the lysates were also probed as indicated. B, HEK293 cells were transiently cotransfected with EphA4 (WT or L920F mutant) fused to C-terminal mTURQ or EYFP fluorescent proteins. Cell lysates were probed by immunoblotting with antibodies to phosphotyrosine (pTyr), the Y779 EphA4 phosphorylation site, and EphA4. C, FRET efficiencies measured for EphA4-mTURQ and EphA4-EYFP, WT, and L920F mutant, in the absence of ligand using the FSI-FRET method. D, mean square error (MSE) values for best-fit oligomerization models ranging from monomers (n = 1) to hexamers (n = 6). The minimum MSE indicates the model that best describes the data. E, FIF measurements performed in HEK293 cells expressing EphA4 WT or L920F. Shown are histograms of the measured molecular brightness (ε), which scales with the oligomer size. The brightness values corresponding to the histogram maxima are indicated by the dotted lines. F, dimeric or oligomeric fractions calculated from the FSI-FRET data are plotted as a function of total receptor concentration for EphA4 WT and L920F. The symbols represent the binned oligomeric fractions and their standard errors. The solid lines represent the best fit curves for monomer–dimer or monomer–oligomer equilibrium. Although lower maximal EphA4 L920F acceptor expression was achieved than for WT (panel C), a complete oligomerization curve was obtained. The dissociation constant (or apparent dissociation constant in the oligomer case) is determined as the receptor concentration at which the oligomeric fraction is 0.5 (50%; see Table 1). G, high-concentration FIF histograms for EphA4 WT and L920F mutant, generated by using the data from E only for receptor concentrations higher than 3000 receptors/μm2. By removing low-concentration data, the monomer populations are not significantly present and thus the maximum of the histogram more accurately indicates receptor oligomer size, ε = 2 (dimer) for EphA4 WT and ε = 3 (trimer) for the L920F mutant. H, FRET efficiencies as a function of acceptor concentration measured for EphA4 WT and L920F in the presence of the ephrinA5-Fc ligand. I, MSE values for EphA4 WT and EphA4 L920F with ephrinA5-Fc. J, FIF histograms for EphA4 WT and L920F mutant in the presence of ephrinA5-Fc. The maxima of the histograms are indicated by dotted lines. K, oligomerization curves for EphA4 WT and the EphA4 L920F mutant in the presence of ephrinA5-Fc (see Table 1). Although lower maximal EphA4 L920F acceptor expression was achieved than for WT (panel H), a complete oligomerization curve was obtained. FRET, Föster resonance energy transfer.