Figure 5.
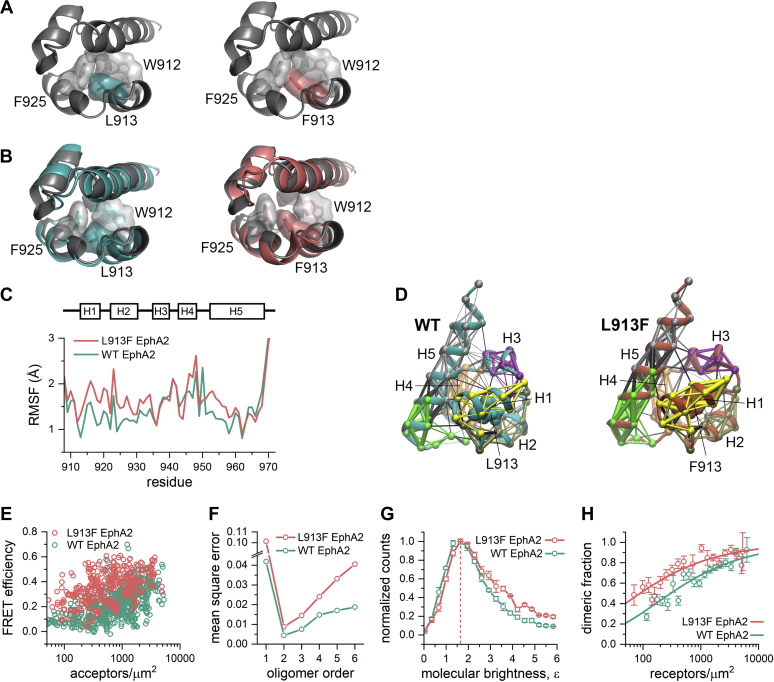
The EphA2 L913F mutation does not promote the formation of higher-order receptor oligomers.A, structure of the EphA2 WT SAM domain (left; PDB ID: 2KSO, chain A) and model of the L913F mutant (right) in ribbon representation with the indicated residues shown as sticks and as a molecular surface. The model, created through a direct substitution of L913 with F, illustrates how W912 and F925 interfere with a phenylalanine at position 913. B, molecular dynamics simulations of the EphA2 WT (left) and L913F (right) SAM domains are aligned to the EphA2 SAM domain crystal structure (gray) by minimizing the backbone RMSD values relative to the crystal structure. C, the RMSF values calculated using the crystal structure coordinates as the reference, are plotted for each residue in the EphA2 WT and L913F SAM domains over the course of the MD simulations, each averaged over three replicates. A schematic of the SAM domain helix positions is shown above the figure. D, dynamic network analysis of the EphA2 WT and L913F SAM domain structures. Nodes, indicated by spheres, highlight the α-carbon atoms and the thickness of the edges (lines connecting the nodes) is proportional to the correlation of atomic motion in space and time. The WT L913 or mutant F913 is rendered with atoms as spheres. Six communities are shown in different colors: community 1 in yellow, community 2 in olive, community 3 in purple, community 4 in orange, community 5 in green, and community 6 in gray. Edges drawn within the same community are colored according to that community and edges drawn between nodes of different communities are colored black. The α-helices (H1 through H5) are indicated. E, comparison of FRET efficiencies measured for the EphA2 WT and L913F mutant in HEK293T cells in the absence of ligand. F, MSE values calculated for EphA2 WT and the L913F mutant. G, FIF histograms showing the molecular brightness distributions for EphA2 WT and L913F in the absence of ligand. The brightness values corresponding to the histogram maxima are indicated by the dotted line. H, the dimeric fractions calculated from the FSI-FRET data for EphA2 L913F are compared with those calculated for EphA2 WT. The symbols represent the binned and averaged dimeric fractions and are shown with the standard errors. The solid lines are the best fit curves for monomer–dimer equilibrium (see Table 1). The data for EphA2 WT in F–H are from (47) and are shown here for comparison. FRET, Föster resonance energy transfer.