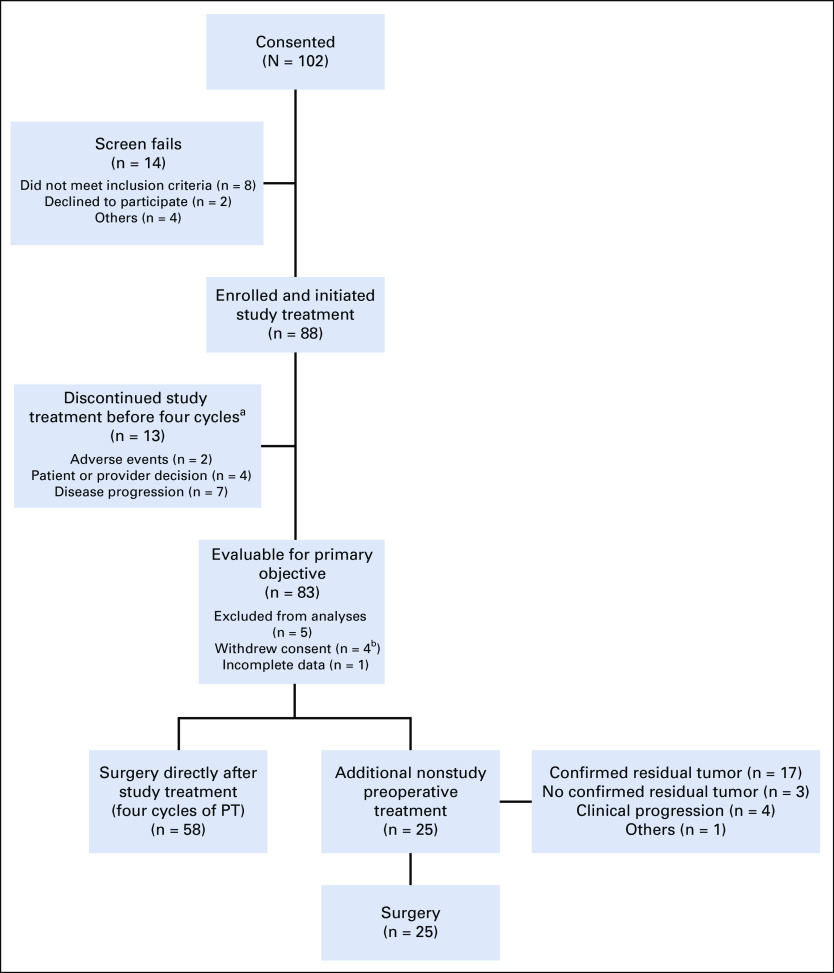
FIG 1.
Study flow diagram. Study treatment = PT. aAll patients remained evaluable. bReasons for withdrawal of consent include one participant refused further participation after C1D1, one participant refused further participation after C2D1, one patient refused further participation after study treatment was completed, and one patient withdrew consent because of unacceptable toxicity. PT, pertuzumab and trastuzumab.