FIG 2.
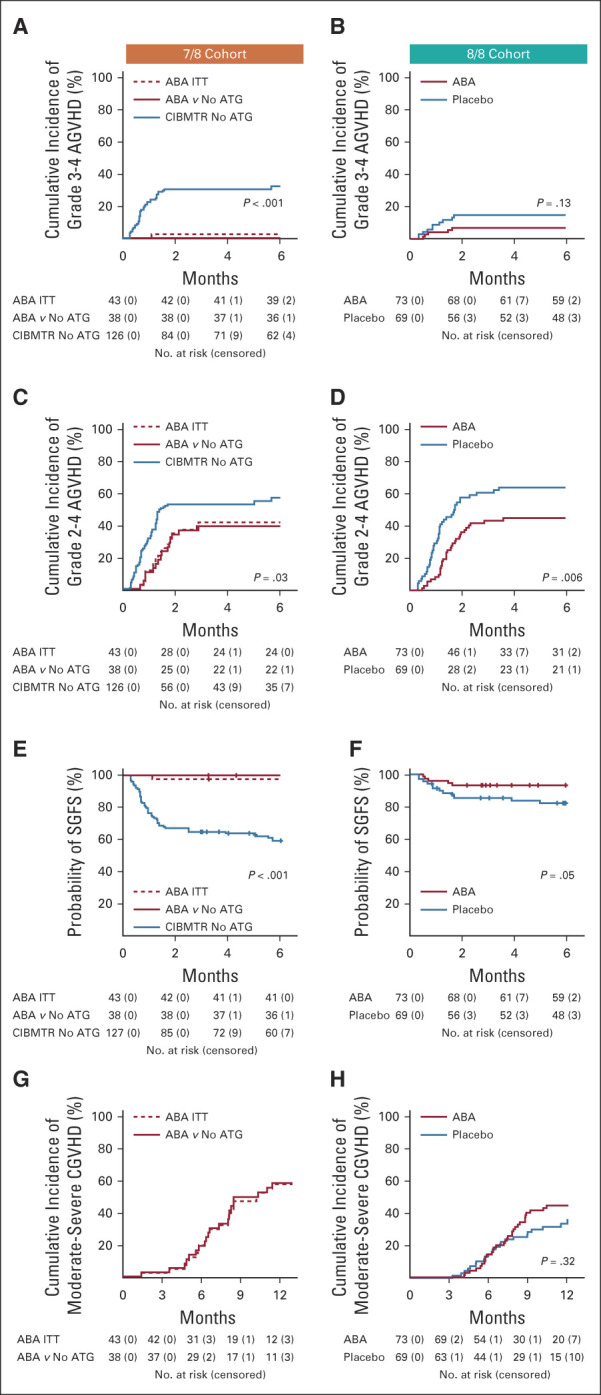
Key GVHD outcomes. (A) 7/8 Cohort, cumulative incidence of grade 3-4 AGVHD. Number of patients at risk and censored are listed below the graph. (B) 8/8 Cohort, cumulative incidence of grade 3-4 AGVHD. Number of patients at risk and censored are listed below the graph. (C) 7/8 Cohort, cumulative incidence of grade 2-4 AGVHD. Number of patients at risk and censored are listed below the graph. (D) 8/8 Cohort, cumulative incidence of grade 3-4 AGVHD. Number of patients at risk and censored are listed below the graph. (E) 7/8 Cohort: cumulative incidence of severe AGVHD-free-survival (SGFS). Number of patients at risk and censored are listed below the graph. (F) 8/8 Cohort: cumulative incidence of SGFS. Number of patients at risk and censored are listed below the graph. (G) 7/8 Cohort: cumulative incidence of moderate-severe CGVHD. Number of patients at risk and censored are listed below the graph. (H) 8/8 Cohort: Cumulative incidence of moderate-severe CGVHD. Number of patients at risk and censored are listed below the graph. 7/8 Cohort: Blue: CIBMTR control cohort. Solid red: 7/8 abatacept cohort, matched to the CIBMTR controls. Dashed red: 7/8 intention-to-treat (ITT) cohort. P values represent comparison of 7/8 abatacept cohort and CIBMTR control cohort. 8/8 Cohort: Blue: placebo. Red: abatacept. ABA, abatacept; AGVHD, acute graft-versus-host disease; ATG, anti-thymocyte globulin; CGVHD, chronic GVHD; CIBMTR, Center for International Blood and Marrow Transplant Research.
