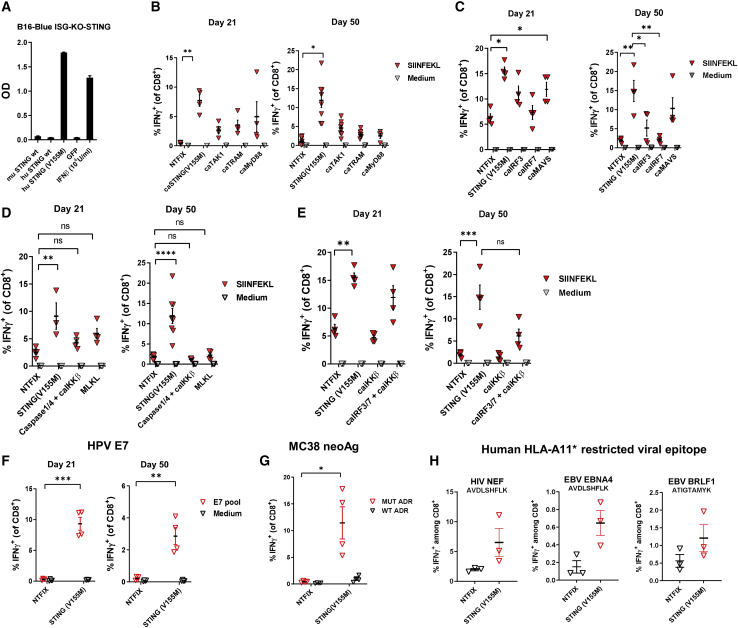
Figure 1.
Comparison of vaccine adjuvant effect among constitutively active variants of mediators of innate immune receptor signaling pathways
(A) B16-Blue ISG-KO-STING IFN-inducible reporter cells were transfected with wild-type and constitutively active STING variants encoded by mRNA or treated with 103 U/mL mouse IFN-β protein as a positive control. At 24 h after transfection, the levels of IRF-induced secreted embryonic alkaline phosphatase (SEAP) were quantitated. C57BL/6 mice were immunized intramuscularly on day 0 and day 14 with LNPs (10 μg/mouse) encapsulating mRNA encoding OVA and test mRNAs. On day 21 and/or day 50, the percentage of SIINFEKL-specific CD8+ T cells in spleens was determined by intracellular staining of IFN-γ, or the percentage of OVA-specific CD8+ T cells in the spleens was determined by flow cytometric analysis of intracellular staining for IFN-γ. (B–E) Results are shown for coformulations with (B) OVA and STINGV155M, caTAK1, caTRAM, or caMyD88; (C) OVA and STINGV155M, caIRF3, caIRF, or caMAVS; (D) OVA and STINGV155M, caspase-1/4+caIKKβ, or MLKL; and (E) OVA and STINGV155M, caIKKβ, or caIRF3+caIRF7+caIKKβ. (F and G) C57BL/6 mice were immunized intramuscularly on day 0 and day 14 with LNPs (10 μg/mouse) encapsulating mRNA encoding HPV16 E7 (F), or tumor neoantigens derived from MC38 murine colon adenocarcinoma cells (G). (H) HLA-A11 transgenic mice were immunized intramuscularly on day 0 and day 14 with LNPs (10 μg/mouse) encapsulating mRNA encoding HLA-A11-restricted viral epitope for HIV NEF, EBV EBNA4, and EBV BRLF1 and STINGV155M. Data are representative of at least two independent experiments. Data plotted are mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.