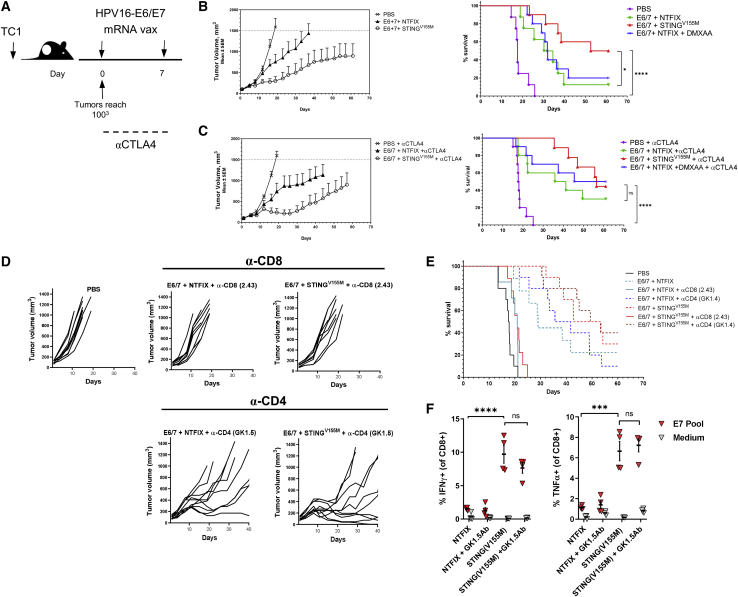
Figure 4.
mRNA-encoded STING adjuvant increases efficacy of HPV E6/E7 cancer vaccine in murine tumor model and requires the presence of CD8+ T cells
(A) C57BL/6 mice (n = 10 per group) were inoculated with 2.5 × 105 TC-1 tumor cells. mRNA vaccine was administered intramuscularly on days 0 and 7 in established tumors (100 mm3). (B and C) Tumor growth and Kaplan-Meier survival curves of mice treated with mRNA vaccine encoding HPV E6/E7 with or without STINGV155M or the mouse STING agonist DMXAA (B); in similar experiments, anti-CTLA-4 (9H10) antibodies were given on days 0, 3, and 6 after the first immunization (C). (D and E) Tumor growth and Kaplan-Meier survival curves of mice were treated as described in (A) with or without depleting antibodies for CD8 or CD4 throughout the duration of the study. (F) C57BL/6 mice were immunized intramuscularly on day 0 and day 14 with mRNA-LNP (10 μg/mouse) coformulated into LNPs with HPV E6/E7 and STINGV155M. Mice were treated with depleting antibodies for CD4 prior to each immunization. On day 21, the percentage of HPV E7-specific CD8+ T cells in spleens was determined by intracellular staining of IFN-γ. Data are representative of at least two independent experiments. Data plotted are mean ± SEM. Statistical significance for survival analyses was calculated using the log-rank test: ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant. Data are representative of two independent experiments.