Abstract
Post-kala-azar dermal leishmaniasis (PKDL) is a chronic, stigmatizing skin condition occurring frequently after apparent clinical cure from visceral leishmaniasis. Given an urgent need for new treatments, we conducted a phase IIa safety and immunogenicity trial of ChAd63-KH vaccine in Sudanese patients with persistent PKDL. LEISH2a (ClinicalTrials.gov: NCT02894008) was an open-label three-phase clinical trial involving sixteen adult and eight adolescent patients with persistent PKDL (median duration, 30 months; range, 6–180 months). Patients received a single intramuscular vaccination of 1 × 1010 viral particles (v.p.; adults only) or 7.5 × 1010 v.p. (adults and adolescents), with primary (safety) and secondary (clinical response and immunogenicity) endpoints evaluated over 42–120 days follow-up. AmBisome was provided to patients with significant remaining disease at their last visit. ChAd63-KH vaccine showed minimal adverse reactions in PKDL patients and induced potent innate and cell-mediated immune responses measured by whole-blood transcriptomics and ELISpot. 7/23 patients (30.4%) monitored to study completion showed >90% clinical improvement, and 5/23 (21.7%) showed partial improvement. A logistic regression model applied to blood transcriptomic data identified immune modules predictive of patients with >90% clinical improvement. A randomized controlled trial to determine whether these clinical responses were vaccine-related and whether ChAd63-KH vaccine has clinical utility is underway.
Keywords: ChAd63-KH vaccine, clinical trial, safety, immunogenicity, PKDL, leishmaniasis, Sudan, transcriptomics
Graphical abstract
Post-kala-azar dermal leishmaniasis (PKDL) is a chronic parasitic disease with limited treatment options. Here, Musa and colleagues report on a phase IIa therapeutic trial of the adenoviral-vectored leishmaniasis vaccine ChAd63-KH. Single-dose vaccination with ChAd63-KH was safe and induced innate and T cell responses in Sudanese patients with PKDL.
Introduction
The World Health Organization recognizes the leishmaniases as some of the most significant global neglected diseases associated with poverty, with over one billion people at risk of infection, with one million new cases and over 20,000 deaths reported each year.1,2 These diseases are caused by infection with one of several species of the protozoan parasite Leishmania and are transmitted by the bite of female phlebotomine sand flies. Clinically, disease may be localized to the site of sand fly bite (cutaneous leishmaniasis [CL]), spread to other skin (disseminated and diffuse leishmaniasis) or mucosal (mucocutaneous leishmaniasis) sites, or may involve systemic organs, notably spleen, liver, and bone marrow (kala azar or visceral leishmaniasis [VL]).3 Post-kala-azar dermal leishmaniasis (PKDL), a chronic skin disease characterized by nodular or macular lesions that start on the face and spread to cover the trunk and arms, may develop in up to 50% of patients previously treated for VL.4 PKDL is thought to play an important role in sustaining the transmission of VL, especially in inter-epidemic periods.4, 5, 6, 7, 8 Although there has been considerable success in reducing the burden of VL in South Asia following the introduction of single-dose liposomal amphotericin B, this drug works less well in other geographic locations, notably East Africa, which then has led to a spate of combination drug trials involving antimonials, miltefosine, paromomycin, and amphotericin B.9 New chemical entities and immune modulators for VL and CL are in the early stages of clinical development but remain untested in the field.9,10 There are currently no effective vaccines for the prevention or treatment of any form of human leishmaniasis.11, 12, 13
First-generation Leishmania vaccines composed of whole killed (autoclaved) parasites often adjuvanted with Bacillus Calmette-Guérin (BCG) were not efficacious in a prophylactic setting14,15 but have shown signs of efficacy as an adjunct to chemotherapy for PKDL16 and American cutaneous leishmaniasis.17,18 Second-generation vaccines that have been evaluated in clinical trials to date have been recombinant poly-protein vaccines, formulated with a variety of lipid-based adjuvants primarily aimed at eliciting CD4+ T cell responses (reviewed in Gillespie et al.,12 Iborra et al.,19 and Moafi et al.20), but these studies have fallen short of demonstrating efficacy in either a prophylactic or therapeutic setting. Leishmania as an intracellular pathogen may also be targeted for immune destruction by effector mechanisms of CD8+ T cells (including IFNγ production and granzyme/granulysin release21), and CD8+ T cell responses have been associated with vaccine-induced protection in animal models.22, 23, 24, 25 Vaccines designed to generate CD8+ T cell responses require a capacity for antigen delivery into the endogenous processing pathway. This is achieved either by facilitating cross-presentation (e.g., using liposomal delivery) or through endogenous protein synthesis (e.g., naked DNA or viral vectors; so called “third-generation” vaccines).
We recently described a third-generation adenovirus-vectored vaccine (ChAd63-KH). ChAd63-KH is based on a well-characterized simian adenovirus backbone (ChAd63), extensively tested in human volunteers and shown to have an excellent safety record.26 ChAd-vectored vaccines induce potent CD8+ and CD4+ T cell responses and antibodies in humans and are amenable to scalable manufacture to good manufacturing practices (GMP). ChAd63-KH encodes two Leishmania antigens, kinetoplastid membrane protein-11 (K; KMP-11) and hydrophilic acylated surface protein B (H; HASPB), both with prophylactic and therapeutic vaccine efficacy when used as monovalent vaccines in pre-clinical animal models (mouse, hamster, or dog).23,24,27 KMP-11 is a highly conserved membrane protein expressed in promastigotes and amastigotes of all Leishmania examined to date and is rich in CD8+ T cell epitopes.22 HASPB is expressed by infective metacyclics and amastigotes28 and has conserved N and C termini flanking polymorphic repeats. These repeats differ in copy number and arrangement across isolates of L. donovani,29 although the functional significance of this is unknown. To increase cross-isolate coverage, we designed a synthetic KH fusion gene for ChAd63-KH, engineered to reflect HASPB repeat-sequence diversity.30 Thus, ChAd63-KH has attributes for a pan-leishmaniasis vaccine.
Results from a first-in-human trial in UK volunteers (ISRCTN: 07766359) indicated that single-dose vaccination with ChAd63-KH was safe, minimally reactogenic, and induced potent innate and cell-mediated immune responses.31 Here, we report on the first use of this vaccine in patients with leishmaniasis. We describe a “window of opportunity” phase IIa clinical trial demonstrating safety and immunogenicity of single-dose ChAd63-KH in Sudanese patients with persistent PKDL.
Results
Study participants and vaccine safety
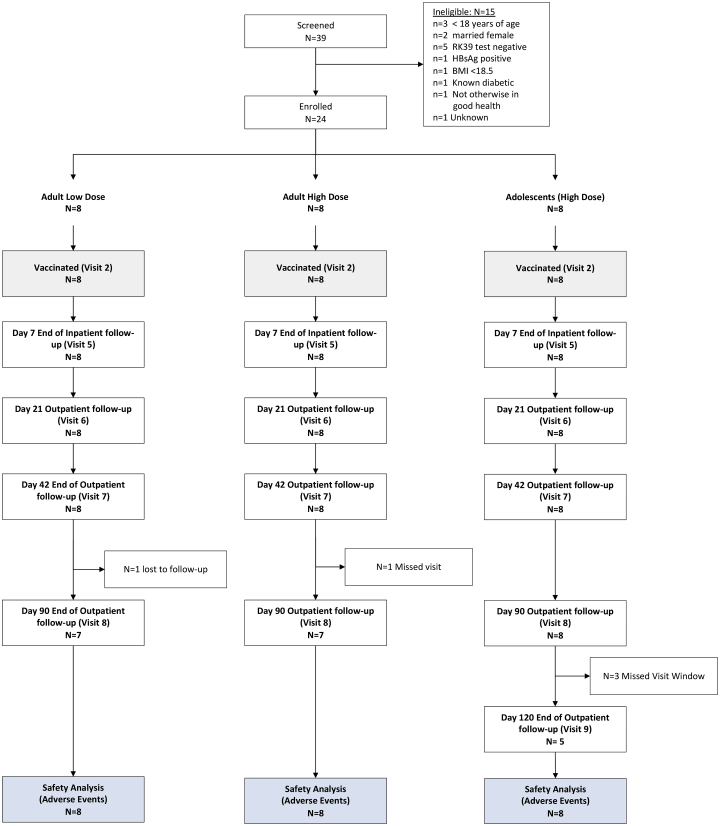
Thirty-nine patients were screened for eligibility between November 2016 and April 2019 (Figure 1), and 24 patients with the demographic and baseline biochemical and hematological characteristics shown in Tables 1 and S1 were enrolled in the study. In each of the adult cohorts, there were six patients with grade 1 PKDL and two patients with grade 2 PKDL. In the adolescent cohort, there were two patients with grade 1 PKDL, five patients with grade 2 PKDL, and one patient with grade 3 PKDL. Median duration of PKDL in the study population was 30 months (range, 6 −180 months). In the adult high-dose, adult low-dose, and adolescent cohorts, respectively, 4 of 8 (50%), 6 of 8 (75%), and 3 of 8 (37.5%) patients had had PKDL for >12 months duration. Twenty-two of all 24 patients were followed up at the scheduled day 90 visit, and 5 of 8 patients in the adolescent cohort were followed up to their day 120 scheduled visit (Figure 1).
Figure 1.
CONSORT diagram for the LEISH2a clinical trial
The CONSORT diagram reports attendance at scheduled inpatient and outpatient visits. Clinical data were also collected for some individuals at additional unscheduled visits, as shown in Figure 3.
Table 1.
Demographics of study participants
| Adults, low dose (n = 8) | Adults, high dose (n = 8) | Adolescents (n = 8) | Total (n = 24) | |
|---|---|---|---|---|
| Sex | ||||
| Male (%) | 7 (87.5) | 8 (100.0) | 6 (75.0) | 21 (87.5) |
| Female (%) | 1 (12.5) | 0 (0.0) | 2 (25.0) | 3 (12.5) |
| Age (years)a | 18.00 (18.00, 19.75) | 23.50 (19.50, 32.00) | 12.50 (12.00, 14.25) | 18.00 (14.75, 23.25) |
| Height (m)a | 1.68 (1.63, 1.71) | 1.73 (1.69, 1.76) | 1.48 (1.37, 1.57) | 1.65 (1.55, 1.72) |
| Weight (kg)a | 54.00 (51.25, 56.75) | 58.50 (56.25, 61.00) | 34.00 (30.50, 41.88) | 52.50 (43.62, 58.25) |
| Duration PKDL (months)a | 30 (6, 57) | 51 (18.75, 102) | 10 (7, 33) | 30 (7, 52.5) |
See Table S1 (sheet 1) for further details.
Data presented as median (Q1, Q3).
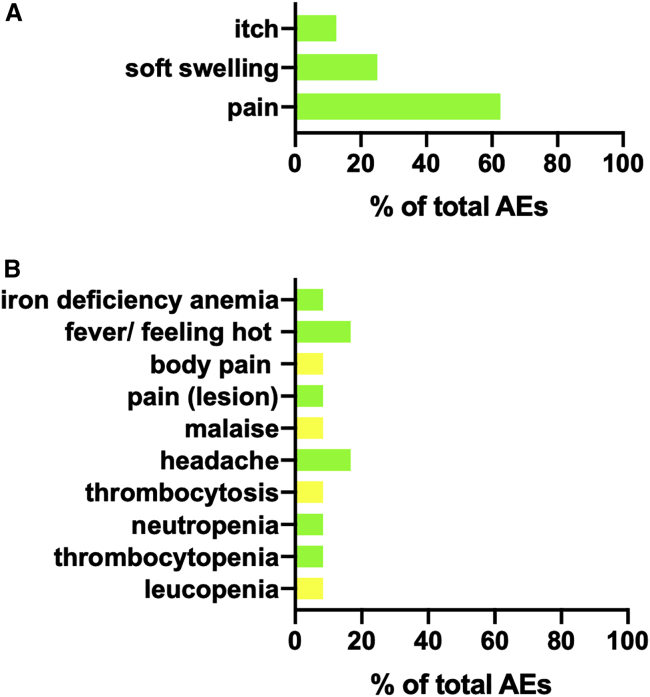
There was a total of 54 (8 local and 46 systemic) adverse events (AEs) reported during the study, of which 20 (8 local and 12 systemic) were considered possibly, probably, or definitely related to vaccination (Figure 2; Table S1). These included local itch (1 patient), soft swelling (2 patients), and pain (5 patients) as well as single cases of systemic malaise, pain, iron-deficiency anemia, and immunological changes (leucopenia, neutropenia, thrombocytopenia, and thrombocytosis). Two patients reported headache and feeling hot. Overall, 7 participants experienced at least one local and 19 experienced at least one systemic AE, with no significant differences in number of events per person between cohorts (median adult low dose: 0 local, 2 systemic; median adult high dose: 0 local, 1.5 systemic; adolescent high dose: 0 local, 1.5 systemic). AEs were limited to grade 1 and 2, with no grade 3, serious adverse events (SAEs), or suspected unexpected serious adverse reactions (SUSARs) reported (Table S1). All local and systemic AEs reported were deemed to be not serious. Medication was not required for any of the local AEs reported, and only two patients had systemic AEs possibly or probably related to vaccination that required medication (one treated with paracetamol for fever, malaise, headache, and body pain and the other treated with ferrous sulfate and folic acid for iron-deficiency anemia).
Figure 2.
Summary of AEs reported in this study
AEs that were possibly, probably, or definitely related to vaccination are shown by category as percentage of total across all three cohorts. (A) Local adverse events (n = 8). (B) Systemic adverse events (n = 12). Grade 1, mild, green bars. Grade 2, moderate, yellow bars.
Clinical follow-up
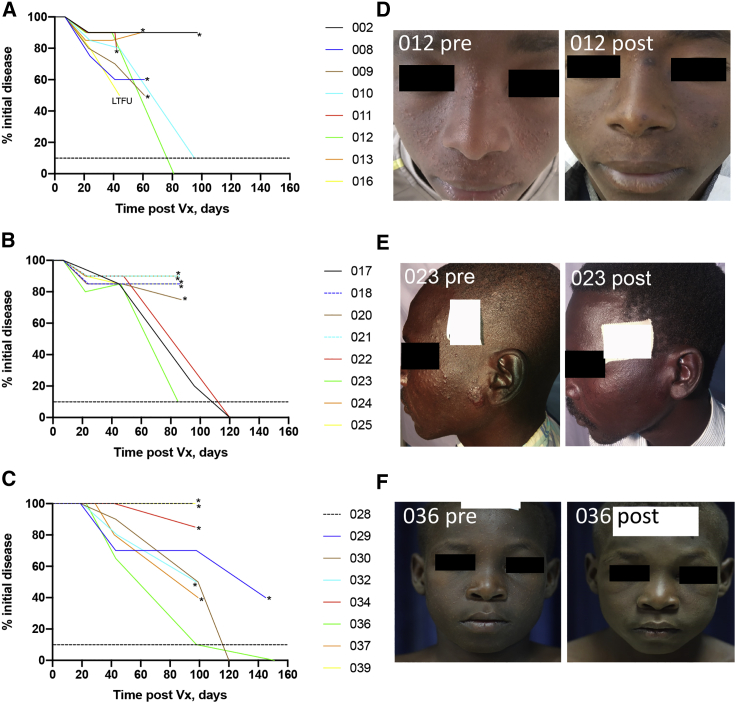
PKDL was subjectively assessed for each individual at each study visit and scored as percentage change in clinical disease relative to time of vaccination (Figure 3). Some patients were unable to attend their formal visits and/or refused treatment at that time due to harvest or schooling commitments and were examined at unscheduled visits. Based on the observation of late cure in some patients in the first two cohorts, the data safety and monitoring board (DSMB) also approved a formal extension of the follow-up period to assess clinical response post vaccination from 42 days to 42–90 days. Based on collective analysis of scheduled and unscheduled visits, 11/23 (47.8%) patients had less than 25% clinical improvement and 5/23 (21.7%) showed clinical improvement of between 40%–60% over the period of follow-up. Patient 016 had 50% improvement of their PKDL at day 42 but was subsequently lost to follow-up. All were treated according to protocol, with the exception of the one patient lost to follow-up. Treatment was not required in 2 of 7 (28.6%) patients in the adult low-dose cohort (both grade 1 PKDL at vaccination; Figures 3A and 3D), 3 of 8 (37.5%) patients in the adult high-dose cohort (two grade 1 PKDL and one grade 2 PKDL at vaccination; Figures 3B and 3E), and 2 of 8 (25%) patients in the adolescent cohort (one grade 2 PKDL and one grade 3 PKDL at vaccination; Figures 3C and 3F) who reached the clinical threshold of >90% improvement in their PKDL. Overall, 7 of 23 (30.4%) patients followed to study completion resolved their PKDL lesions without the need for chemotherapy.
Figure 3.
Clinical outcome for LEISH2a
Data are presented for each patient as percentage of initial PKDL disease over time post vaccination, normalized to the day of vaccination. (A) Low-dose adult cohort. (B) High-dose adult cohort. (C) High-dose adolescent cohort. Asterisks indicate patient received conventional treatment with AmBisome. LTFU, lost to follow-up. This patient was excluded from the assessment of overall cure rate but included here for completion, as by the time of LTFU the patient had shown a clinical response of 50%. Dotted line represents 90% clinical improvement. (D–F) Representative patient photographs taken pre-vaccination and at the last follow-up visit are provided for cohort 1 (patient 012; D), cohort 2 (patient 023; E), and cohort 3 (patient 036; F). Patient 012 and 036 had widespread small papular lesions pre-vaccination that became flattened in appearance and in the case of patient 012 also showed areas of re-/hyper-pigmentation. Patient 023 also had numerous small papular lesions as well as more pronounced nodular lesions (e.g., near the ear) pre-vaccination, with resolution post vaccination. White boxes are placed to hide patient-identifying stickers and retain anonymity.
Whole-blood transcriptome prior to and after vaccination
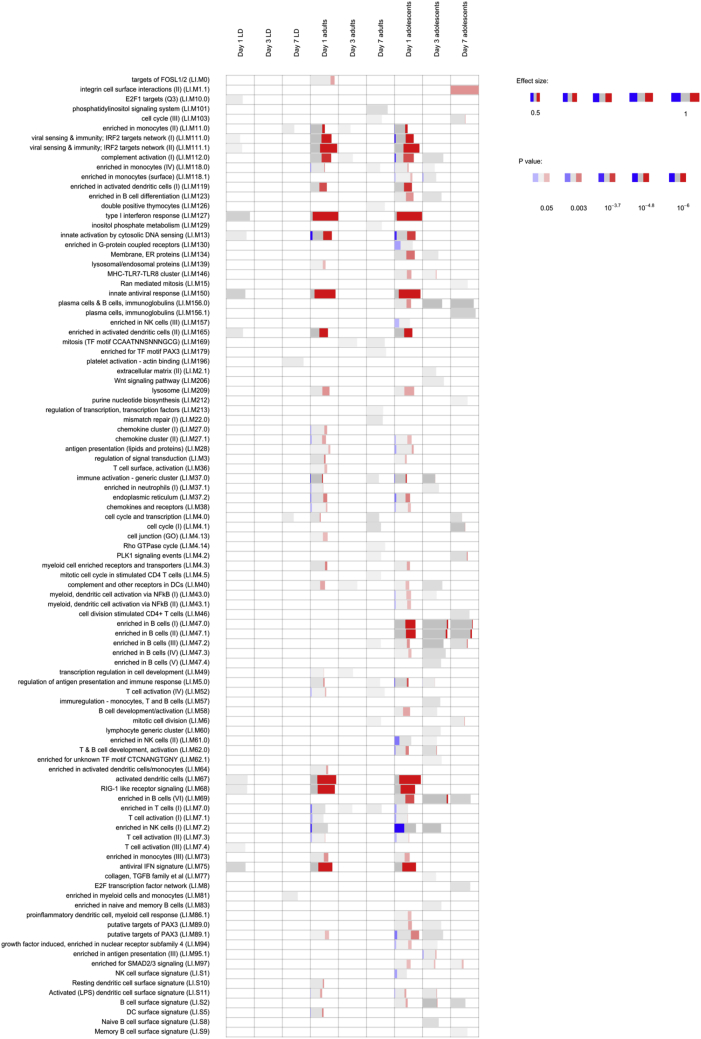
Immune responses in the patient cohort were assessed using whole-blood transcriptomic analysis (WBTA), comparing pre-vaccination blood to blood taken at 1-, 3-, and 7-days post vaccination. Differentially expressed genes were identified (Table S2) and used to identify transcriptional modules significantly associated with vaccination (Figure 4; Table S3). These data identified three key features of the response. First, modules associated with an anti-viral signature and with dendritic cell activation showed a marked dose dependence, being minimal in patients receiving low-dose vaccine. Second, there was a near equivalence of these innate responses in adults and adolescents receiving high-dose vaccine. Third, modules associated with B cell responses were prominent only in adolescents. As approximately 30% of patients resolved their PKDL over the follow-up period, we sought to identify potential predictors of resolution. We used a logistic regression model to identify potential predictors of patients with >90% clinical resolution, using the Z scores associated with the blood transcription modules (Figure 4). This analysis revealed 11 modules (predictive modules sheet in Table S3), focused on monocyte and dendritic cell attributes, identified to have highest predictive value for clinical response. Among these 11 modules, two were also identified as differentially expressed: LI.M139 (lysosomal/endosomal proteins) and LI.M118.0 (enriched in monocytes) (Table S3).
Figure 4.
Whole-blood transcriptomic analysis (WBTA) of patient responses to vaccination with ChAd63-KH
WBTA was conducted using the Ion AmpliSeq Transcriptome Human Gene Expression Kit. Each column represents a different time point (days 1, 3, and 7) after vaccination in the three study groups (low-dose adults, high-dose adults, high-dose adolescents). Significantly enriched immune-related modules were identified applying the CERNO test on the adjusted p value-ranked lists of genes generated by DeSeq2 (see Table S2 for module gene lists). Modules are represented by bars in which the proportion of significantly upregulated and downregulated genes is shown in red and blue, respectively. The gray portion of the bar represents genes that are not significantly differentially regulated. The significance of module activation is proportional to the intensity of the bar, while the effect size is proportional to its width.
We also analyzed differentially expressed genes identified at day 1 post vaccination with 7.5 × 1010 viral particles (v.p.) (Table S3) by Ingenuity Pathway Analysis and for gene set enrichment (using EnrichR). Comparative pathway analysis of differentially expressed genes in adults (374 UP; 108 DOWN) and adolescents (510 UP; 439 DOWN) showed a high degree of concordance in predicted upstream regulators (e.g., IFNG [Z score of 9.169 versus 8.847 for adults versus adolescents], IFNA2 [Z score of 7.834 versus 7.925], and the transcription factor IRF7 [Z score of 7.424 versus 7.35]). In EnrichR, we similarly identified enriched Gene Ontology (GO) and Reactome pathways related mainly to interferon type I and II signaling, anti-viral response, myeloid cells, phago-lysosomal functions, and class I major histocompatibility complex (MHC)-mediated antigen processing and presentation (Table S4), in keeping with the results of the modular analysis described above. Thus, patients with PKDL appear to mount effective innate cellular responses to ChAd63-KH.
Finally, we also interrogated our pre-vaccination transcriptomic data as a means of evaluating whether there were any differences in gene expression at baseline between those patients who cleared their PKDL lesions at the end of the study versus those who required drug treatment. No differentially expressed genes were identified with false discovery rate (FDR) set at 5%.
CD8+ T cell response following vaccination
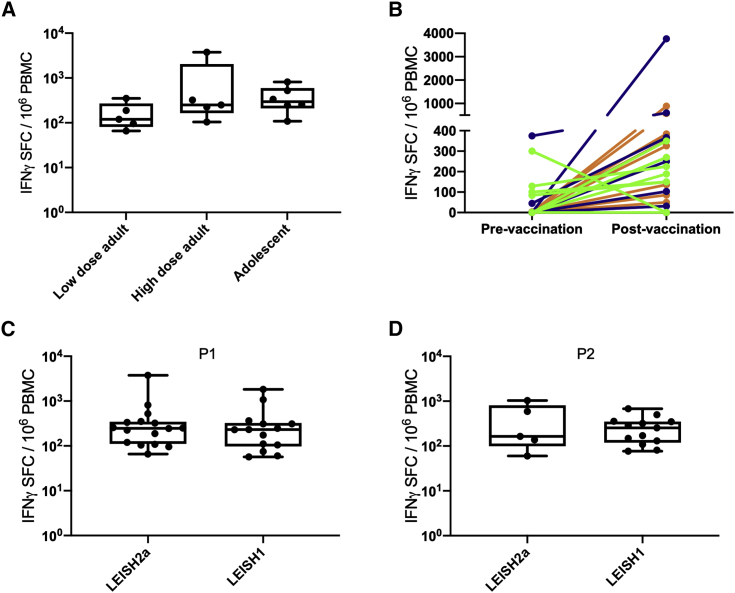
IFNγ production by CD8+ T cells was measured using ELISpot following re-stimulation with peptide pools. The frequency of CD8+ T cells producing IFNγ specifically in response to KMP-11 (pool 1) was not significantly different between high- and low-dose vaccinated adults or between adult and adolescent cohorts (Figure 5A). Overall ELISpot frequency of responders to KMP-11 after vaccination was 66.6% (16/24), with peak responses in responders ranging from 66–3,766 spot forming cells (SFC)/million peripheral blood mononuclear cells (PBMCs; mean, 485; 95% CI, 8.8–962) (Figures 5B and S1). Ten patients had responses to KMP-11 prior to vaccination of greater than 50 SFC/106 PBMCs, which were of variable magnitude and either declined or increased post vaccination. Four of the low-dose adult cohort had immune responses to KMP-11 measured after treatment, with 2/4 showing some increase compared to their pre-vaccination response (Figure S1). Responses were comparable in frequency and magnitude to those seen previously in healthy UK volunteers (Figure 5C). Cells from adolescent patients were also evaluated for response to the N-terminal of HASPB (pool 2; 5/8 responders; mean, 397; 95% CI, −114–908), again showing comparable results to that seen in UK volunteers (Figures 5D and S2). These data indicate that PKDL patients respond with similar vigor to the ChAd63-vectored vaccine antigens as previously observed in healthy UK volunteers.31 Given the small sample size, we cannot draw any firm conclusions from these data about the relationship between magnitude or specificity of the CD8+ T cell response and clinical outcome.
Figure 5.
CD8+ T cell response to vaccination with ChAd63-KH
PBMCs from patients collected from d7-d90 post vaccination were stimulated with peptide pools representing the entire KMP-11 sequence (P1) and the HASPB N terminus (P2). The number of IFNγ-producing cells/million PBMCs was determined by ELISpot. (A) Peak response by cohort to P1 after subtraction of unstimulated background and any pre-vaccination response. (B) Pre-vaccination and peak post vaccination response per patient to P1 for low-dose adult (green), high-dose adult (blue), and high-dose adolescents (orange). (C and D) Comparison between patients in this trial (LEISH2a) and healthy UK volunteer responses (LEISH1) for response to P1 (C) and P2 (D). Data for LEISH1 are taken from Osman et al.31 Box-and-whisker plots indicate median, 25th−75th quartiles, mix/max values, and individual patient data points.
Discussion
Previous studies in Sudan pioneered the use of immunotherapy with first-generation vaccines (autoclaved L. major/alum + BCG) in combination with sodium stibogluconate,16,32 but use of a vaccine as monotherapy in PKDL patients has not been previously reported. In this phase IIa dose-escalation, age de-escalation clinical trial we have demonstrated that ChAd63-KH is both safe and immunogenic in Sudanese PKDL patients, setting the scene for further studies aimed at evaluating efficacy in a therapeutic setting.
The rationale for using vaccines for therapeutic benefit or for post-exposure prophylaxis is not new and has been evaluated for a variety of chronic viral infections, including HIV33 and HPV34 as well as in cancer.35 While some chronic viral infections may subvert immunity to such an extent to pose a barrier to therapeutic vaccination,36 recent evidence suggests that such limitations to vaccine efficacy can be overcome.37 Evidence also suggests leishmaniasis patients may respond well to therapeutic vaccination. For example, protective immunity is readily reactivated after drug cure;11 virally vectored antigen delivery generates effector CD8+ T cells and therapeutic benefit in rodents,30,38 and there have been encouraging data from human immunochemotherapy trials in leishmaniasis patients.16,17
PKDL in Sudan has a complex natural history.5, 6, 7 In most cases it emerges within 3–6 months of the cessation of treatment for VL, reminiscent of an immune reactivation disease,5 although cases also occur during and even in the absence of prior VL.4,39 In a study of the natural history of PKDL in 134 children younger than 14 years,40 84% of patients showed spontaneous remission of their disease with a mean (standard deviation [SD]) of 9.7 (4.7) months. For the remaining 16% (21 patients), duration of PKDL was 16.6 (5.5) months, with over half of these cases showing either no change or worsening of PKDL during the first 12 months. Grade or severity of PKDL did not appear to influence the duration of PKDL, and, unless disease was very severe, these patients did not require treatment.40 Although formal time-to-event data are not available, and other age groups have not been studied systematically, current clinical practice in Sudan is based on the premise that patients with persistent PKDL for 6 months or longer duration are not expected to rapidly self-resolve their lesions, and such patients are therefore provided with the standard of care, a protracted course of liposomal amphotericin B (AmBisome; 2.5 mg/kg/day for 20 days). Hence, as for other studies evaluating new drug regimens for PKDL,16,41,42 patients with PKDL of greater than 6 months duration were enrolled for this study (median duration of PKDL of 30 months), with the expectation of a relatively stable disease over the short window of follow-up. With the caveat that our present study contains no control arm for self-cure, we are encouraged by the finding that over a 3- to 4-month period, approximately one-third of patients resolved their PKDL lesions in the absence of further treatment, with a further 25% showing some clinical improvement. The extended and variable time frames over which clinical improvements in PKDL were observed was perhaps not surprising, given the highly heterogeneous nature of PKDL in this study population. A randomized placebo-controlled trial (RCT) of ChAd63-KH in persistent PKDL patients in Sudan is currently underway (ClinicalTrials.gov: NCT03969134) to determine whether the clinical improvements observed in the current study are vaccine-related and, if so, whether biomarkers can be identified to predict the likelihood of a clinical response to vaccination. Given the significant costs, patient discomfort, and risks associated with current treatment options, should this level of clinical response be shown to be due to vaccination, it would represent a major benefit for patients and a welcome new treatment option, particularly if biomarkers were available to stratify patients for drug or vaccine treatment. While only ∼20% of patients in Sudan present with persistent PKDL, in South Asia persistent disease reflects the norm, and PKDL patients represent a significant risk to the regional VL elimination campaign.43, 44, 45 The results from the current study also provide a clear incentive to evaluate therapeutic vaccination with ChAd63-KH as a tool for the management of PKDL cases in South Asia.44
Similar to healthy UK volunteers receiving the same vaccine, PKDL patients showed no unusual vaccine-induced responses, in keeping with their general state of health. Indeed, the WBTA indicated that PKDL patients respond with a vigorous innate immune response, qualitatively similar to that seen in healthy UK volunteers31 in terms of GO term enrichment and predicted upstream regulators of gene expression. A direct quantitative comparison is not possible, however, due to the use of different platforms for WBTA analysis (Ion AmpliSeq Transcriptome Human Gene Expression Kit in this study versus RNA-seq in Osman et al.31). Module analysis suggested that adolescents with PKDL may generate more pronounced B cell responses to ChAd63-KH than adults, though the functional significance of this remains to be determined, and the small sample size and variable demographics suggest this finding needs confirmation with a larger sample size and through more detailed phenotypic and functional analysis of the B cell response. Nevertheless, stronger humoral responses in adolescents have been observed previously with other vaccines (e.g., the quadrivalent HPV vaccine).46,47 Through a logistic regression model, we also identified a small number of modules with predictive power for identifying patients reaching the clinical endpoint of >90% improvement. Of note, module LI.M139 contains only 11 genes encoding endo/lysosomally located proteins and enzymes, namely the scavenger receptor CD68; the catabolic enzyme acid glucosidase (GAA); cathepsins B, D, H, and S (involved in various aspects of antigen presentation and myeloid cell activation48); proteins associated with cholesterol transport (NPC2) and glycolipid catabolism (PSAP); proteins involved in endo/lysosomal protein sorting (AP1S2 and SORT1); and the transporter SLC11A1 (a polymorphic divalent metal ion transporter associated with natural resistance to Leishmania and other intracellular pathogens and enhanced antigen presentation49,50). It remains to be determined to what extent this vaccine-induced molecular signature relates to vaccine efficacy or is reflective of underlying immunological or genetic differences in the response to vaccination in those patients destined to self-resolve their PKDL. The latter situation may be viewed as somewhat analogous to the association between leishmanin skin test reaction, self-resolution, and/or response to therapy observed in PKDL patients.4,16,40
We found that cellular immune responses as measured here using IFNγ ELISpot were on par with those seen with healthy UK volunteers. While recognizing that this is not a comparable healthy endemic control group, the data nevertheless suggest that PKDL patients do not have underlying immunosuppression. No strong dose-response relationship was noted across cohorts, as observed in other studies conducted over similar limited dose ranges.26,31 We observed that a small number of patients had a pre-existing response to KMP-11 and the N-terminal of HASPB, and an apparent boosting as well as apparent loss of response was noted over time post vaccination. Equally, in the small number of patients where it was measured, there was an apparent increase in response to these antigens after treatment. While this might reflect the broad range of kinetics observed in our study of this vaccine in healthy UK volunteers,31 it cannot be ruled out that the responses observed reflect changes in the underlying immune response to Leishmania in these patients over time. The immunological follow-up of patients in the placebo arm of our ongoing RCT should help provide answers to these questions.
This study does have some limitations. First, the immunology of Sudanese PKDL is complex and still poorly understood,5,6,51,52 though it is reasonably well-established that active disease is associated with high levels of interleukin (IL)-10 that gives way to enhanced IFNγ and a restoration of skin test reactivity following resolution.5,51 Although the purpose of this study was not to examine heterogeneity within the immune status of patients with PKDL and its relationship to disease outcome, we did have the opportunity to examine baseline gene transcription in whole blood of patients enrolled in this study. We did not, however, identify any differentially expressed genes that were related to patient outcome. This result is perhaps not surprising, given the relatively small sample size under study, and further analysis of this type may be more fruitful in the context of our ongoing RCT. In addition, the methodology chosen for assessment of the response to vaccine antigens pre and post vaccination (ELISpot; the gold standard for assessment of adenoviral vaccine-induced T cell responses) as well as limitations in the yield of PBMCs meant that we could not directly measure additional CD4+ or CD8+ T cell-derived T helper 1 (Th1) and Th2-related cytokines.
In conclusion, this study demonstrating the safety and immunogenicity of ChAd63-KH in PKDL patients represents an important milestone in the development of a therapeutic vaccine as an additional tool for PKDL patient management and more broadly encourages further exploration of therapeutic adenovirus-vectored vaccines for other infectious diseases.
Methods and methods
Ethics statement
The study was approved by the Review Committees of the Institute of Endemic Diseases, University of Khartoum, the Sudan National Medicines and Poisons Board, and the Department of Biology, University of York. LEISH2a was sponsored by the University of York. The study was conducted according to the principles of the current revision of the Declaration of Helsinki 2008 and International Conference on Harmonisation (ICH) guidelines for good clinical practice (GCP; CPMP/ICH/135/95) and was registered as ClinicalTrials.gov: NCT02894008. All participants provided written informed consent before enrollment.
Study design and participants
LEISH2a was an open-label three-phase study designed to evaluate the safety (primary endpoint), clinical response, and immunogenicity (secondary endpoints) of the investigational vaccine ChAd63-KH in 24 patients with persistent PKDL of greater than 6 months duration. As this is the first time ChAd63-KH has been administered to humans with ongoing persistent PKDL, this sample size allows initial assessment of safety outcomes and determination of the magnitude of the outcome measures, rather than aiming to obtain statistical significance. The number of participants in each part of the study is typical for early vaccine studies and is considered sufficient to achieve the objectives of the study. Participants were patients diagnosed with persistent PKDL aged between 18–50 years (adults) or 12–16 years (adolescents). The diagnosis of a case of persistent PKDL was based on a typical distribution of the skin rash for a duration of 6 months or more, a temporal relationship to treated kala azar, a reactive serology test, and exclusion of other skin condition. PKDL lesions were defined per protocol as grade 1 (scattered maculopapular or nodular lesions, mainly around the mouth), grade 2 (dense maculopapular or nodular rash covering most of the face and extending to chest, back, upper arms and legs), or grade 3 (dense maculopapular or nodular rash covering most of the body, including hands and feet.).53 Inclusion criteria included: uncomplicated PKDL of >6 months duration; availability for the duration of the study; otherwise good health as determined by medical history, physical examination, results of screening tests, and the clinical judgment of a medically qualified clinical investigator; negative for malaria on blood smear; judged able and likely to comply with all study requirements; willing to undergo screening for HIV, hepatitis B, and hepatitis C; for females only, willing to undergo urinary pregnancy tests on the day of screening, on the day of vaccination (prior to vaccination), and 7 and 42 days after vaccination. Exclusion criteria included: mucosal or conjunctival PKDL; treatment for PKDL within 21 days; negative for antibodies in the RK39 strip test; receipt of a live attenuated vaccine within 60 days or other vaccine within 14 days of screening; administration of immunoglobulins and/or any blood products within the 3 months preceding the planned administration of the vaccine candidate; history of allergic disease or reactions likely to be exacerbated by any component of the vaccine or a history of severe or multiple allergies to drugs or pharmaceutical agents; any history of severe local or general reaction to vaccination; for females only, pregnancy, less than 12 weeks postpartum, lactating, or willingness/intention to become pregnant during the study and for 3 months following vaccination; seropositive for hepatitis B surface antigen (HBsAg) or hepatitis C (antibodies to HCV); any clinically significant abnormal finding on screening biochemistry or hematology blood tests or urinalysis; any confirmed or suspected immunosuppressive or immunodeficient state, including HIV infection; asplenia; recurrent, severe infections and chronic (more than 14 days) immunosuppressant medication within the past 6 months; tuberculosis, leprosy, or malnutrition (malnutrition in adults defined as a BMI < 18.5, and in adolescents [12–17 years] as a Z score cutoff value of < −2 SD); any other significant disease, disorder, or finding increasing risk to the volunteer, likely to influence the result of the study, or the volunteer’s ability to participate.
Participants were recruited from an endemic area in Gedaref state, Sudan, and all study procedures were conducted at the Professor El-Hassan’s Centre for Tropical Medicine, Dooka, Sudan. Monitoring of the study was performed under contract by ClinServ (http://www.clinserv.net).
Vaccine and study procedures
The clinical vaccine lot (B0004) was manufactured by Advent (Pomezia, Italy) as described in detail elsewhere.31 The vaccine is a sterile aqueous buffered solution containing ChAd63-KH at a concentration of 7.5 × 1010 v.p./mL. ChAd63-KH was administered as a single dose in 1mL volume intramuscularly into the deltoid muscle. The first eight adult volunteers received 1 × 1010 v.p., and the subsequent eight adult volunteers received 7.5 × 1010 v.p. These doses were previously assessed for safety and immunogenicity in a healthy volunteer phase I trial31 and are typical of the doses administered in other adenovirus vaccine trials. Based on a review of the clinical data, eight adolescents were vaccinated with 7.5 × 1010 v.p. A cautious stepwise approach was taken during vaccination, with the first participant at each dose being followed for 21 days before vaccination of subsequent participants in that cohort. Patients were monitored in the hospital for 7 days post vaccination and thereafter as outpatients on days 21, 42, 90, and 120 post vaccination (depending on cohort). An independent DSMB meeting was held at the end of each cohort to review data and provide advice to the sponsor regarding continuation of the trial. Clinical and biochemical test abnormalities were graded according to the protocol, based on NIH guidelines. Treatment for AEs was provided as required. The natural history of PKDL in Sudan indicates that patients with disease persistent for greater than 6 months are unlikely to self-cure.40 A final endpoint for clinical response was scheduled to be made between day 42 and day 90 (see Results). Patients with less than 75% improvement were offered standard treatment with AmBisome (2.5 mg/kg/day for 20 days), those with between 75%–90% improvement were offered conservative treatment or AmBisome, and those with greater than 90% clinical improvement were deemed to not require further treatment. Standard treatment with AmBisome (20 days; 2.5 mg/kg/day) was provided in the hospital, and patients were confirmed as clinically cured at the end of treatment. Some patients defaulted from scheduled visits and were evaluated and treated at unscheduled visits based on their availability. Decisions to treat and evaluation of PKDL were performed by two experienced clinicians based on a subjective assessment of overall clinical improvement in the patient’s skin condition. Photographs of PKDL lesions were independently reviewed to confirm degree of clinical improvement.
WBTA
Whole-blood samples (2.5 mL) were collected into PAXGene tubes immediately prior to vaccination and at 1-, 3-, and 7-days post vaccination. All reagents and equipment for these analyses were supplied by Thermo Fisher Scientific and processes carried out per manufacturers’ protocols, unless otherwise stated. Total RNA was extracted using the PAXgene Blood RNA kit (PreAnalytiX, QIAGEN). RNA was quantified using the Qubit 2.0 Fluorometer with the RNA HS Assay Kit. ∼50 ng of total RNA was used to construct sequencing libraries with the Ion AmpliSeq Transcriptome Human Gene Expression Kit. Libraries were barcoded, purified with 2.5× Agencourt AMPure XP Magnetic Beads (Beckman Coulter), and then quantified using Ion Library TaqMan Quantitation Kit on a QuantStudio 5. Libraries were diluted to a concentration of about 50 pmol and pooled in groups of 8 for sequencing on Ion PI Chips. Chips were loaded using the Ion Chef System and the Ion PI Hi-Q Chef Kit. Sequencing was performed on an Ion Proton Sequencer using Ion PI Hi-Q Sequencing 200 Kit. Data has been deposited in the Gene Expression Omnibus (GEO) repository .
Differential gene expression analysis was performed using DeSeq2.54 After count data normalization, differential gene expression analysis was performed using pooled day 0 data from the three study cohorts as the baseline for all contrasts. Enrichment of blood transcription modules at each time point in the different groups was assessed with the tmod R package,55 using as an input the lists of differentially expressed genes ranked by the p value after multiple test correction, as computed by DeSeq2. Significance of module enrichment was assessed using the CERNO statistical test (a modification of Fisher’s combined probability test) and corrected for multiple testing using the Benjamini-Hochberg correction. In order to identify the modules with highest correlation to the clinical response, the Z score of each module was used to train a 1-dimensional Logistic Regression model. After 100 bootstraps, the modules were ranked according to the average prediction score. Data analyses were performed by python scripts using the scikit-learn python library.56 Gene set enrichments were performed in EnrichR,57 and pathway analysis was conducted using IPA (QIAGEN, https://digitalinsights.qiagen.com/products/ingenuity-pathway-analysis).58
Assessment of vaccine-induced immunity
Ex vivo re-stimulation of frozen PBMCs to elicit vaccine-induced CD8+ T cell responses was performed (at days 0, 21, 42, and 90) using Multiscreen IP ELISpot plates (Millipore), human IFNγ SA-APL antibody kits (Mabtech), and BCIP-NBT-plus chromogenic substrate (Moss) as previously described.31 Peptide re-stimulation was restricted to peptide pools corresponding to the entire KMP-11 sequence and the N-terminal conserved domain of HASPB1 (pools 1 and 231) due to limitations in cells obtained from patients. Peptides were 8- to 11-mer truncated sets as fully described in Osman et al.,31 largely restricting recognition to CD8+ T cells. Responses to medium-only negative controls were subtracted, and responses >50 SFC/million above the pre-vaccination response were regarded as positive. Antibody responses will be reported at a later date due to inaccessibility of trial samples at the current time due to COVID-19.
Statistical analysis
Twenty-four was chosen as an appropriate sample size for this phase IIa vaccine study to evaluate safety outcomes and explore the magnitude of outcome measures. It was not formally derived to show significant differences from any comparisons. All baseline data were summarized descriptively. Continuous measures are reported as averages (n, mean, SDs, median, interquartile range (IQR), min, max), and categorical data are reported as counts and percentages. Number of local and systemic AEs per participant are presented as median, minimum, maximum, and IQR. The median number of AEs per participant (separately for local and systemic events) was compared between groups using the Mann-Whitney U test (three comparisons for each type of event). Analyses were performed using Stata v1659 and R v3.5.3.60 ELISpot data were evaluated for normality using the D’Agnostino and Pearson test and compared using non-parametric Kolmogorov-Smirnov test. All analysis was performed in GraphPad Prism for MacOS v8.4.1.
Data availability
Transcriptomic data have been deposited in the Gene Expression Omnibus (GEO) repository with accession number GEO: GSE156645 and are available from: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE156645.
Acknowledgments
The clinical trial was funded by a Wellcome Trust Translation Award (WT108518; https://wellcome.org). Additional support for immunological studies was provided by a Wellcome Trust Senior Investigator Award (WT104726 to P.M.K.) and the TRANSVAC2 program supported by the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement no. 730964 (TNA1802-02; https://www.transvac.org). The funders played no role in study design or decision to publish.
Author contributions
Conceptualization, A.M.M., C.J.N.L., E.A.G.K., P.M.K., R.G., M.B., and T.A.; methodology, A.M.M., A.M.L., B.M.Y., C.J.N.L., E.A.G.K., F.S., M.O., P.M.K., S.F., and R.W.; investigation, A.E.A.M., A.K., A.M.L., B.M.Y., C.J.N.L., E.A.G.K., F.S., M.A.A.A., M.O., L.M., P.M.K., and S.F.; writing – original draft, P.M.K.; writing – review & editing, A.K., A.M.M., A.M.L., C.J.N.L., E.A.G.K., M.O., P.M.K., R.G., and T.A.; funding acquisition, A.M.M., C.J.N.L., E.A.G.K., P.M.K., R.G., M.B., and T.A.; resources, A.M.M., C.J.N.L., E.A.G.K., and P.M.K.; supervision, A.M.M., C.J.N.L., E.A.G.K., and P.M.K.
Declaration of interests
C.J.N.L., P.M.K., and T.A. are co-authors of a patent protecting the gene insert used in candidate vaccine ChAd63-KH (Europe 10719953.1; India 315101). The authors otherwise declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.03.020.
Contributor Information
Paul M. Kaye, Email: paul.kaye@york.ac.uk.
Ahmed M. Musa, Email: musaam2003@yahoo.co.uk.
Supplemental information
Sheet 1 provides demographic characteristics and biochemistry and haematology data at baseline. Sheet 2 summarises local and systemic AE number by cohort. Sheets 3 and 4 summarise AEs by relatedness to study intervention. Sheet 5 lists all reported AEs and grade.
Sheet 1 contains count data for each patient at each time point examined. Sheet 2 contains patient descriptors and read depth and rate of target detection. Sheets 3 and 4 contain DE gene lists for adults (sheet 3) and adolescents (sheet 4) comparing those vaccinated with 7.5X1010 vp ChAd63-KH to pre-vaccination baseline.
Sheet 1 contains genes associated with each immune module used in this study. Sheet 2 contains genes predicted to be associated with clinical response based on analysis using all modules or only modules DE at p>0.05. Remaining sheets provide lists of the significantly enriched modules for each cohort at each time point and /or dose.
Sheets contain output from EnrichR for GO Biological Processes 2018, Cellular Component 2018, Reactome 2016 and WikiPathways 2019 for DE genes at day 1 post vaccination in adult high dose and adolescent high dose cohorts.
References
- 1.Alvar J., Vélez I.D., Bern C., Herrero M., Desjeux P., Cano J., Jannin J., den Boer M., WHO Leishmaniasis Control Team Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation . 2019. Leishmaniasis.https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis [Google Scholar]
- 3.Davies C.R., Kaye P., Croft S.L., Sundar S. Leishmaniasis: new approaches to disease control. BMJ. 2003;326:377–382. doi: 10.1136/bmj.326.7385.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zijlstra E.E., Musa A.M., Khalil E.A., el-Hassan I.M., el-Hassan A.M. Post-kala-azar dermal leishmaniasis. Lancet Infect. Dis. 2003;3:87–98. doi: 10.1016/s1473-3099(03)00517-6. [DOI] [PubMed] [Google Scholar]
- 5.Khalil E.A., Khidir S.A., Musa A.M., Musa B.Y., Elfaki M.E., Elkadaru A.M., Zijlstra E., El-Hassan A.M. Post-Kala-Azar Dermal Leishmaniasis: A Paradigm of Paradoxical Immune Reconstitution Syndrome in Non-HIV/AIDS Patients. J. Trop. Med. 2013;2013:275253. doi: 10.1155/2013/275253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukhopadhyay D., Dalton J.E., Kaye P.M., Chatterjee M. Post kala-azar dermal leishmaniasis: an unresolved mystery. Trends Parasitol. 2014;30:65–74. doi: 10.1016/j.pt.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musa A.M., Khalil E.A., Younis B.M., Elfaki M.E., Elamin M.Y., Adam A.O., Mohamed H.A., Dafalla M.M., Abuzaid A.A., El-Hassan A.M. Treatment-based strategy for the management of post-kala-azar dermal leishmaniasis patients in the Sudan. J. Trop. Med. 2013;2013:708391. doi: 10.1155/2013/708391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zijlstra E.E., Alves F., Rijal S., Arana B., Alvar J. Post-kala-azar dermal leishmaniasis in the Indian subcontinent: A threat to the South-East Asia Region Kala-azar Elimination Programme. PLoS Negl. Trop. Dis. 2017;11:e0005877. doi: 10.1371/journal.pntd.0005877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alves F., Bilbe G., Blesson S., Goyal V., Monnerat S., Mowbray C., Muthoni Ouattara G., Pécoul B., Rijal S., Rode J., et al. Recent Development of Visceral Leishmaniasis Treatments: Successes, Pitfalls, and Perspectives. Clin. Microbiol. Rev. 2018;31:e00048-18. doi: 10.1128/CMR.00048-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao S.P.S., Barrett M.P., Dranoff G., Faraday C.J., Gimpelewicz C.R., Hailu A., Jones C.L., Kelly J.M., Lazdins-Helds J.K., Mäser P., et al. Drug Discovery for Kinetoplastid Diseases: Future Directions. ACS Infect. Dis. 2019;5:152–157. doi: 10.1021/acsinfecdis.8b00298. [DOI] [PubMed] [Google Scholar]
- 11.Alvar J., Croft S.L., Kaye P., Khamesipour A., Sundar S., Reed S.G. Case study for a vaccine against leishmaniasis. Vaccine. 2013;31(Suppl 2):B244–B249. doi: 10.1016/j.vaccine.2012.11.080. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie P.M., Beaumier C.M., Strych U., Hayward T., Hotez P.J., Bottazzi M.E. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine. 2016;34:2992–2995. doi: 10.1016/j.vaccine.2015.12.071. [DOI] [PubMed] [Google Scholar]
- 13.Reed S.G., Coler R.N., Mondal D., Kamhawi S., Valenzuela J.G. Leishmania vaccine development: exploiting the host-vector-parasite interface. Expert Rev. Vaccines. 2016;15:81–90. doi: 10.1586/14760584.2016.1105135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noazin S., Khamesipour A., Moulton L.H., Tanner M., Nasseri K., Modabber F., Sharifi I., Khalil E.A., Bernal I.D., Antunes C.M., Smith P.G. Efficacy of killed whole-parasite vaccines in the prevention of leishmaniasis: a meta-analysis. Vaccine. 2009;27:4747–4753. doi: 10.1016/j.vaccine.2009.05.084. [DOI] [PubMed] [Google Scholar]
- 15.Noazin S., Modabber F., Khamesipour A., Smith P.G., Moulton L.H., Nasseri K., Sharifi I., Khalil E.A., Bernal I.D., Antunes C.M., et al. First generation leishmaniasis vaccines: a review of field efficacy trials. Vaccine. 2008;26:6759–6767. doi: 10.1016/j.vaccine.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 16.Musa A.M., Khalil E.A., Mahgoub F.A., Elgawi S.H., Modabber F., Elkadaru A.E., Aboud M.H., Noazin S., Ghalib H.W., El-Hassan A.M., Leishmaniasis Research Group/Sudan Immunochemotherapy of persistent post-kala-azar dermal leishmaniasis: a novel approach to treatment. Trans. R. Soc. Trop. Med. Hyg. 2008;102:58–63. doi: 10.1016/j.trstmh.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Machado-Pinto J., Pinto J., da Costa C.A., Genaro O., Marques M.J., Modabber F., Mayrink W. Immunochemotherapy for cutaneous leishmaniasis: a controlled trial using killed Leishmania (Leishmania) amazonensis vaccine plus antimonial. Int. J. Dermatol. 2002;41:73–78. doi: 10.1046/j.1365-4362.2002.01336.x. [DOI] [PubMed] [Google Scholar]
- 18.Mayrink W., Magalhaes P.A., Michalick M.S., da Costa C.A., Lima Ade.O., Melo M.N., Toledo V.P., Nascimento E., Dias M., Genaro O., et al. Immunotherapy as a treatment of American cutaneous leishmaniasis: preliminary studies in Brazil. Parassitologia. 1992;34:159–165. [PubMed] [Google Scholar]
- 19.Iborra S., Solana J.C., Requena J.M., Soto M. Vaccine candidates against leishmania under current research. Expert Rev. Vaccines. 2018;17:323–334. doi: 10.1080/14760584.2018.1459191. [DOI] [PubMed] [Google Scholar]
- 20.Moafi M., Rezvan H., Sherkat R., Taleban R. Leishmania Vaccines Entered in Clinical Trials: A Review of Literature. Int. J. Prev. Med. 2019;10:95. doi: 10.4103/ijpvm.IJPVM_116_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barman H., Walch M., Latinovic-Golic S., Dumrese C., Dolder M., Groscurth P., Ziegler U. Cholesterol in negatively charged lipid bilayers modulates the effect of the antimicrobial protein granulysin. J. Membr. Biol. 2006;212:29–39. doi: 10.1007/s00232-006-0040-3. [DOI] [PubMed] [Google Scholar]
- 22.Basu R., Roy S., Walden P. HLA class I-restricted T cell epitopes of the kinetoplastid membrane protein-11 presented by Leishmania donovani-infected human macrophages. J. Infect. Dis. 2007;195:1373–1380. doi: 10.1086/513439. [DOI] [PubMed] [Google Scholar]
- 23.Das S., Freier A., Boussoffara T., Das S., Oswald D., Losch F.O., Selka M., Sacerdoti-Sierra N., Schönian G., Wiesmüller K.H., et al. Modular multiantigen T cell epitope-enriched DNA vaccine against human leishmaniasis. Sci. Transl. Med. 2014;6:234ra56. doi: 10.1126/scitranslmed.3008222. [DOI] [PubMed] [Google Scholar]
- 24.Stäger S., Alexander J., Kirby A.C., Botto M., Rooijen N.V., Smith D.F., Brombacher F., Kaye P.M. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nat. Med. 2003;9:1287–1292. doi: 10.1038/nm933. [DOI] [PubMed] [Google Scholar]
- 25.Stäger S., Rafati S. CD8(+) T cells in leishmania infections: friends or foes? Front. Immunol. 2012;3:5. doi: 10.3389/fimmu.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Hara G.A., Duncan C.J., Ewer K.J., Collins K.A., Elias S.C., Halstead F.D., Goodman A.L., Edwards N.J., Reyes-Sandoval A., Bird P., et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J. Infect. Dis. 2012;205:772–781. doi: 10.1093/infdis/jir850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno J., Nieto J., Masina S., Cañavate C., Cruz I., Chicharro C., Carrillo E., Napp S., Reymond C., Kaye P.M., et al. Immunization with H1, HASPB1 and MML Leishmania proteins in a vaccine trial against experimental canine leishmaniasis. Vaccine. 2007;25:5290–5300. doi: 10.1016/j.vaccine.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maclean L.M., O’Toole P.J., Stark M., Marrison J., Seelenmeyer C., Nickel W., Smith D.F. Trafficking and release of Leishmania metacyclic HASPB on macrophage invasion. Cell. Microbiol. 2012;14:740–761. doi: 10.1111/j.1462-5822.2012.01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zackay A., Nasereddin A., Takele Y., Tadesse D., Hailu W., Hurissa Z., Yifru S., Weldegebreal T., Diro E., Kassahun A., et al. Polymorphism in the HASPB repeat region of East African Leishmania donovani strains. PLoS Negl. Trop. Dis. 2013;7:e2031. doi: 10.1371/journal.pntd.0002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maroof A., Brown N., Smith B., Hodgkinson M.R., Maxwell A., Losch F.O., Fritz U., Walden P., Lacey C.N., Smith D.F., et al. Therapeutic vaccination with recombinant adenovirus reduces splenic parasite burden in experimental visceral leishmaniasis. J. Infect. Dis. 2012;205:853–863. doi: 10.1093/infdis/jir842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osman M., Mistry A., Keding A., Gabe R., Cook E., Forrester S., Wiggins R., Di Marco S., Colloca S., Siani L., et al. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: First-in-human trial of ChAd63-KH. PLoS Negl. Trop. Dis. 2017;11:e0005527. doi: 10.1371/journal.pntd.0005527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghalib H., Modabber F. Consultation meeting on the development of therapeutic vaccines for post kala azar dermal leishmaniasis. Kinetoplastid Biol. Dis. 2007;6:7. doi: 10.1186/1475-9292-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moretti S., Cafaro A., Tripiciano A., Picconi O., Buttò S., Ensoli F., Sgadari C., Monini P., Ensoli B. HIV therapeutic vaccines aimed at intensifying combination antiretroviral therapy. Expert Rev. Vaccines. 2020;19:71–84. doi: 10.1080/14760584.2020.1712199. [DOI] [PubMed] [Google Scholar]
- 34.Garbuglia A.R., Lapa D., Sias C., Capobianchi M.R., Del Porto P. The Use of Both Therapeutic and Prophylactic Vaccines in the Therapy of Papillomavirus Disease. Front. Immunol. 2020;11:188. doi: 10.3389/fimmu.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mougel A., Terme M., Tanchot C. Therapeutic Cancer Vaccine and Combinations With Antiangiogenic Therapies and Immune Checkpoint Blockade. Front. Immunol. 2019;10:467. doi: 10.3389/fimmu.2019.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swadling L., Halliday J., Kelly C., Brown A., Capone S., Ansari M.A., Bonsall D., Richardson R., Hartnell F., Collier J., et al. Highly-Immunogenic Virally-Vectored T-cell Vaccines Cannot Overcome Subversion of the T-cell Response by HCV during Chronic Infection. Vaccines (Basel) 2016;4:E27. doi: 10.3390/vaccines4030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borducchi E.N., Cabral C., Stephenson K.E., Liu J., Abbink P., Ng’ang’a D., Nkolola J.P., Brinkman A.L., Peter L., Lee B.C., et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature. 2016;540:284–287. doi: 10.1038/nature20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joshi T., Rodriguez S., Perovic V., Cockburn I.A., Stäger S. B7-H1 blockade increases survival of dysfunctional CD8(+) T cells and confers protection against Leishmania donovani infections. PLoS Pathog. 2009;5:e1000431. doi: 10.1371/journal.ppat.1000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ismail A., Khalil E.A., Musa A.M., El Hassan I.M., Ibrahim M.E., Theander T.G., El Hassan A.M. The pathogenesis of post kala-azar dermal leishmaniasis from the field to the molecule: does ultraviolet light (UVB) radiation play a role? Med. Hypotheses. 2006;66:993–999. doi: 10.1016/j.mehy.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 40.Musa A.M., Khalil E.A., Raheem M.A., Zijlstra E.E., Ibrahim M.E., Elhassan I.M., Mukhtar M.M., El Hassan A.M. The natural history of Sudanese post-kala-azar dermal leishmaniasis: clinical, immunological and prognostic features. Ann. Trop. Med. Parasitol. 2002;96:765–772. doi: 10.1179/000349802125002211. [DOI] [PubMed] [Google Scholar]
- 41.Musa A.M., Khalil E.A., Mahgoub F.A., Hamad S., Elkadaru A.M., El Hassan A.M. Efficacy of liposomal amphotericin B (AmBisome) in the treatment of persistent post-kala-azar dermal leishmaniasis (PKDL) Ann. Trop. Med. Parasitol. 2005;99:563–569. doi: 10.1179/136485905X514127. [DOI] [PubMed] [Google Scholar]
- 42.Younis B.M., Mohammed H.A.A., Dafalla M.M.M., Adam A.O.A., Elamin M.Y., Musa A.M., et al. Cure of post kala-azar dermal leishmaniasis with paromomycin/sodium stibogluconate combination: a proof of concept International. J. Res. Med. Sci. 2015;3:16–21. [Google Scholar]
- 43.Le Rutte E.A., Zijlstra E.E., de Vlas S.J. Post-Kala-Azar Dermal Leishmaniasis as a Reservoir for Visceral Leishmaniasis Transmission. Trends Parasitol. 2019;35:590–592. doi: 10.1016/j.pt.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mondal D., Bern C., Ghosh D., Rashid M., Molina R., Chowdhury R., Nath R., Ghosh P., Chapman L.A.C., Alim A., et al. Quantifying the Infectiousness of Post-Kala-Azar Dermal Leishmaniasis Toward Sand Flies. Clin. Infect. Dis. 2019;69:251–258. doi: 10.1093/cid/ciy891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sengupta R., Chaudhuri S.J., Moulik S., Ghosh M.K., Saha B., Das N.K., Chatterjee M. Active surveillance identified a neglected burden of macular cases of Post Kala-azar Dermal Leishmaniasis in West Bengal. PLoS Negl. Trop. Dis. 2019;13:e0007249. doi: 10.1371/journal.pntd.0007249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Block S.L., Nolan T., Sattler C., Barr E., Giacoletti K.E., Marchant C.D., Castellsagué X., Rusche S.A., Lukac S., Bryan J.T., et al. Protocol 016 Study Group Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118:2135–2145. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 47.Dobson S.R., McNeil S., Dionne M., Dawar M., Ogilvie G., Krajden M., Sauvageau C., Scheifele D.W., Kollmann T.R., Halperin S.A., et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309:1793–1802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 48.Jakoš T., Pišlar A., Jewett A., Kos J. Cysteine Cathepsins in Tumor-Associated Immune Cells. Front. Immunol. 2019;10:2037. doi: 10.3389/fimmu.2019.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braliou G.G., Kontou P.I., Boleti H., Bagos P.G. Susceptibility to leishmaniasis is affected by host SLC11A1 gene polymorphisms: a systematic review and meta-analysis. Parasitol. Res. 2019;118:2329–2342. doi: 10.1007/s00436-019-06374-y. [DOI] [PubMed] [Google Scholar]
- 50.Kaye P.M., Patel N.K., Blackwell J.M. Acquisition of cell-mediated immunity to Leishmania. II. LSH gene regulation of accessory cell function. Immunology. 1988;65:17–22. [PMC free article] [PubMed] [Google Scholar]
- 51.Zijlstra E.E. The immunology of post-kala-azar dermal leishmaniasis (PKDL) Parasit. Vectors. 2016;9:464. doi: 10.1186/s13071-016-1721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zijlstra E.E. Biomarkers in Post-kala-azar Dermal Leishmaniasis. Front. Cell. Infect. Microbiol. 2019;9:228. doi: 10.3389/fcimb.2019.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zijlstra E.E., el-Hassan A.M. Leishmaniasis in Sudan. Visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2001;95(Suppl 1):S27–S58. doi: 10.1016/s0035-9203(01)90218-4. [DOI] [PubMed] [Google Scholar]
- 54.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiner J., 3rd, Domaszewska T. tmod: an R package for general and multivariate enrichment analysis. PeerJ Preprints. 2016;4:e2420v1. [Google Scholar]
- 56.Pedregosa F., Varoquaux G., Gramfort A., Michel V., Thirion B., Grisel O., Blondel M., Prettenhofer P., Weiss R., Dubourg V., et al. Scikit-learn: Machine Learning in Python. Journal of Machine Learning. 2011;12:2825–2830. [Google Scholar]
- 57.Kuleshov M.V., Jones M.R., Rouillard A.D., Fernandez N.F., Duan Q., Wang Z., Koplev S., Jenkins S.L., Jagodnik K.M., Lachmann A., et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–W97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krämer A., Green J., Pollard J., Jr., Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.StataCorp . StataCorp; 2019. Stata Statistical Software: Release 16. [Google Scholar]
- 60.R Development Core Team . R Foundation for Statistical Computing; 2011. R: A Language and Environment for Statistical Computing. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sheet 1 provides demographic characteristics and biochemistry and haematology data at baseline. Sheet 2 summarises local and systemic AE number by cohort. Sheets 3 and 4 summarise AEs by relatedness to study intervention. Sheet 5 lists all reported AEs and grade.
Sheet 1 contains count data for each patient at each time point examined. Sheet 2 contains patient descriptors and read depth and rate of target detection. Sheets 3 and 4 contain DE gene lists for adults (sheet 3) and adolescents (sheet 4) comparing those vaccinated with 7.5X1010 vp ChAd63-KH to pre-vaccination baseline.
Sheet 1 contains genes associated with each immune module used in this study. Sheet 2 contains genes predicted to be associated with clinical response based on analysis using all modules or only modules DE at p>0.05. Remaining sheets provide lists of the significantly enriched modules for each cohort at each time point and /or dose.
Sheets contain output from EnrichR for GO Biological Processes 2018, Cellular Component 2018, Reactome 2016 and WikiPathways 2019 for DE genes at day 1 post vaccination in adult high dose and adolescent high dose cohorts.
Data Availability Statement
Transcriptomic data have been deposited in the Gene Expression Omnibus (GEO) repository with accession number GEO: GSE156645 and are available from: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE156645.