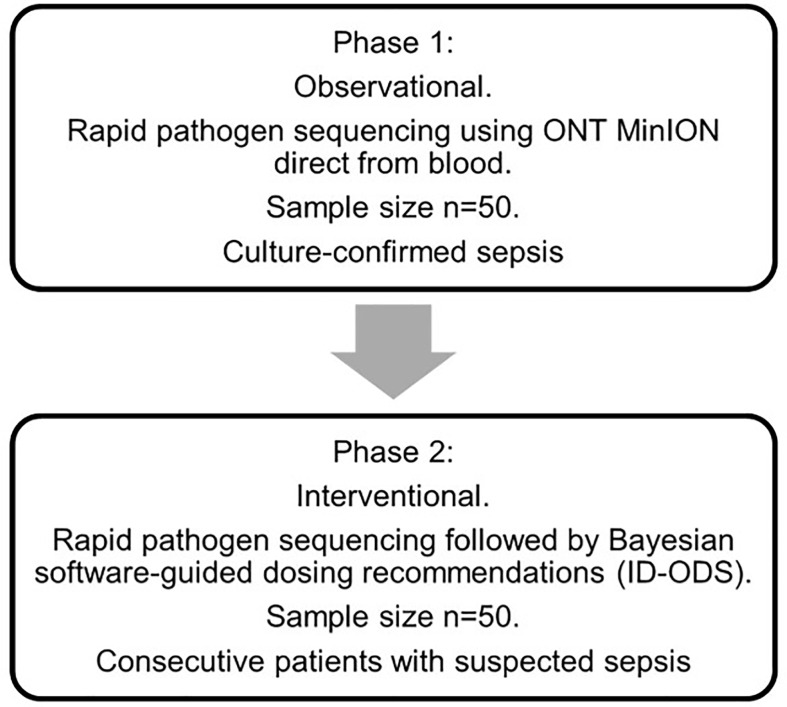
Figure 1.
Study flow diagram. Phase 1: In an initial observational phase of the study, participants will be recruited to a diagnostic accuracy study of MinION nanopore pathogen sequencing. The sample size for this phase of the study is 50 patients with blood culture-confirmed sepsis admitted to ICU. Phase 2: In Phase 2, consecutive patients with suspected sepsis admitted to ICU will undergo MinION nanopore pathogen sequencing integrated with personalised antibiotic therapy using a combination of Bayesian dosing software (ID-ODS™) and measured antibiotic plasma concentrations. A senior ICU pharmacist/clinician at each site will lead this software-guided intervention of antimicrobial dose optimisation. All dosing regimens will be checked by both the senior ICU pharmacist and attending ICU consultant prior to prescription. The final decision regarding the use of the optimised dosing of antibiotics will remain at the discretion of the attending ICU consultant. Software-guided dosing will continue until either: 1) the study antibiotic(s) have been ceased by the treating clinician, 2) the patient is discharged from ICU, 3) after 5 days of study antibiotic therapy. If antibiotic therapy is still required thereafter, dosing will be guided by the treating clinician. Adherence to dosing strategies informed by the dosing software will be supported by the use of senior ICU pharmacists trained in the use of this software-guided approach to antimicrobial dose optimisation. All dosing regimens will be checked by both the senior ICU pharmacist and attending ICU consultant prior to prescription, to ensure appropriateness and safety.