Abstract
Objectives
Sarcopenia is relatively common in rheumatoid arthritis (RA) patients. Thicknesses of the quadriceps muscle and fat are easily measured by ultrasound (US) and are known to be related to skeletal muscle mass and fat mass, respectively.
Methods
Eighty-four patients enrolled in the prospective correlation research of sarcopenia, skeletal muscle, and disease activity in rheumatoid arthritis study (UMIN000023744) underwent US examinations of anterior thigh muscle thickness (MT) and fat thickness (FT). Muscle and body fat (BF) mass were also examined by a body composition analyzer. Whether MT and FT were related to sarcopenia and obesity was examined.
Results
MT was significantly lower in RA patients with sarcopenia than in those without (23.8 vs 28.2 mm, P = 0.001). MT was related to sarcopenia (men: r = 0.56, P = 0.02, women: r = 0.32, P = 0.01). The cut-off value of MT for sarcopenia was 24.7 mm in men and 19.7 mm in women on receiver operating characteristic curve analyses. FT was correlated with BF percentage (%BF; men: r = 0.66, P < 0.01, women: r = 0.62, P < 0.001), which was estimated by 2.04xFT+8.53 in men and 1.2xFT+17.42 in women by a simple linear regression model. This means that FT ≥ 8.1 mm in men and FT ≥ 14.6 mm in women indicated obesity.
Conclusions
US examination of the anterior thigh was useful to detect sarcopenia and obesity in RA patients.
Keywords: Obesity, Prospective observational study, Rheumatoid arthritis, Sarcopenia, Ultrasound
1. Introduction
The quality of life (QOL) of patients with rheumatoid arthritis (RA) has improved with progress in treatment, such as biological and targeted synthetic disease-modifying antirheumatic drugs (DMARDs) [1], [2], [3]. The incidence of fragility fractures and falls is higher in RA patients than in healthy individuals [[4], [5], [6]], and their risk increases in aged patients. Due to the increase in the aged population rate, there is growing interest in sarcopenia, defined [7] as low muscle mass, muscle power, and muscle function, and frailty, defined [8] as degradation of physical status. We previously reported that the prevalence of sarcopenia was 28% [9], and that of frailty was 18.9% in patients with RA, and frailty was significantly increased due to poor disease control in a prospective, cohort study [10]. It is difficult for aged people to recover from sarcopenia. Therefore, early intervention is necessary to prevent sarcopenia. The European Working Group on Sarcopenia in Older People (EWGSOP) suggests that aged people should undergo routine screening [11].
RA patients have deformities and destruction of hand, wrist, hip, knee, ankle, and foot joints by disease. Therefore, it is difficult to use the grip test and gait speed, which are diagnostic criteria of sarcopenia, since RA patients easily fulfill the criteria. It is necessary to use some evaluation tool or test that is not affected by deformity and the joint destruction caused by the disease.
Measurement of muscle mass is required to diagnose sarcopenia by both the EWGSOP [11] and the Asia Working Group for Sarcopenia (AWGS) [12]. The measurement of muscle mass and body fat is done using computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US), dual-energy X-ray absorptiometry (DXA), and body composition analyzers. CT, MRI, and DXA are not suitable for screening because the equipment requires a large space and long examination time [13]. On the other hand, US has the advantage of portability, rapid measurement, widespread availability, and lack of radiation exposure. Muscle mass measured by US correlated with that measured by DXA, CT, and MRI [14].
Obesity is an independent risk factor for cardiovascular events [15] and metabolic syndrome [16]. A relationship between sarcopenia and obesity has been reported. Decreased muscle mass leads to glucose dysregulation and increased insulin resistance, and its continuation contributes to the onset of obesity [17]. Screening for obesity is important to decrease cardiovascular events and reduce sarcopenic obesity and metabolic syndrome.
The use of US has gradually become widespread at clinical sites to diagnose and evaluate disease activity in patients with RA. Our hypothesis was that US is useful for screening for sarcopenia and obesity by measuring thigh muscle thickness and fat thickness in patients with RA.
2. Methods
2.1. Participants
A prospective, observational study was started in 2016 to investigate correlations among sarcopenia, locomotive syndrome, and RA disease activity, as the correlation research of sarcopenia, skeletal muscle, and disease activity in rheumatoid arthritis (CHIKARA) study [9]. This study was registered with the UMIN Clinical Trials Registry [http://www.umin.ac.jp/ctr/] (UMIN000023744). Correlations among sarcopenia, obesity, and muscle and fat thicknesses of the anterior thigh on ultrasound were investigated by cross-sectional analysis using the 2-year follow-up data of the CHIKARA study.
This study included 100 consecutive patients with RA (78 women, 22 men) seen in general clinical practice at our hospital. All patients with musculoskeletal diseases, neurological diseases, malignancy, heart failure, severe renal dysfunction, or surgically implanted metal were excluded from this study. Patients taking drugs affecting body composition, such as diuretics, were also excluded. All patients with RA were ≥ 20 years old and fulfilled the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria [18]. The details of the study protocol and inclusion criteria were presented in our previous report [9]. At 2-year follow-up, 16 cases (9 cases, personal choice; 3 cases, death; 3 cases, relocation; 1 case, entered retirement home) dropped out. Therefore, 84 patients with RA were available for this analysis. The ethics committee of Osaka City General Hospital approved the study protocol (Approval number: 1505019). Written, informed consent for participation in this study and publication of identifying information/images in an online-access publication was obtained from all patients prior to enrolment, in accordance with the Declaration of Helsinki.
2.2. Measuring thigh muscle and fat thicknesses by ultrasound
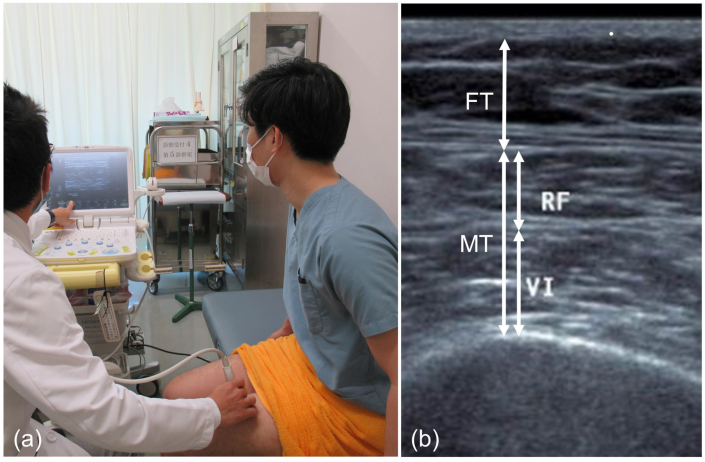
The measuring method was reported previously [19]. Patients sat on a chair with hip and knee flexion at 90° each. The upper edges of the patella and greater trochanter were palpated. Their midpoints were measured by a measuring tape. Ultrasound scans were performed using the HI VISION Noblus device and a linear probe transducer 18-5 MHz (Hitachi Aloka Medical Ltd., Tokyo, Japan). The probe was placed perpendicular at the midpoint between the upper edges of the patella and greater trochanter of the anterior thigh, and transverse images were recorded by B-mode. Muscle thickness (MT) was defined as the distance between the anterior fascia of the rectus femoris muscle and the posterior fascia of the vastus intermedius muscle. Fat thickness (FT) was defined as the distance between the anterior and posterior subcutaneous fat (Fig. 1). MT and FT of both thighs were measured by one clinician (M.T.), and the average value was calculated and used in the analysis. The intraclass correlation coefficient of intraobserver agreement for MT and FT were 0.897 and 0.913, respectively.
Fig. 1.
The ultrasound machine is always ready to measure muscle and fat thicknesses in the consultation room. The ultrasound probe is set at the midpoint between the upper edges of the patella and greater trochanter (a). The muscle and fat thicknesses are evaluated in a transverse image (b). Muscle thickness (MT) is defined as the distance between the anterior fascia of the rectus femoris muscle (RF) and the posterior fascia of the vastus intermedius muscle (VI). Fat thickness (FT) is defined as the distance between the anterior and posterior subcutaneous fat.
2.3. Diagnosis of sarcopenia and obesity
Body compositions were measured using a bioelectrical impedance analyzer (MC-780A; TANITA, Tokyo, Japan). Weight, body mass index, muscle mass, body fat mass, total body water, estimated bone mass, and basal metabolic rate for the total body and regions (arms, legs, and trunk) were measured by this device. Gait speed was determined by a 3-m walk test, and grip strength was measured for each hand using a digital hand-held isokinetic dynamometer (TKK-5401; Takei Scientific Instruments, Niigata, Japan). Sarcopenia was diagnosed using the AWGS criteria [12]. The cut-off values used were < 0.8 m/s for gait speed and < 26 kg in men and < 18 kg in women for grip strength. Sarcopenia was diagnosed when the appendicular skeletal mass index (ASMI) from the bioelectrical impedance analysis (BIA) method was < 7.0 kg/m2 in men and < 5.7 kg/m2 in women. Body fat percentage (%BF) was also measured by this device. Obesity was defined as %BF ≥ 25% and 35% in men and women, respectively [20,21].
2.4. Clinical assessments of rheumatoid arthritis
The rheumatologist of each patient determined the treatment strategy based on the treat-to-target concept [22] during the observation period. All patients completed a self-administered questionnaire about general health status, activities of daily living (ADL), and falls and fractures over a year of observation. Laboratory examinations included C-reactive protein (CRP), matrix metalloproteinase 3 (MMP3), and the erythrocyte sedimentation rate (ESR). RA activity was measured as the disease activity score (DAS) composite of the ESR and the 28-joint score (DAS28-ESR) [23]. Functional status was also measured in patients with RA based on modified health assessment questionnaire (mHAQ) scores [24].
2.5. Statistical analysis
The CHIKARA study's sample of 100 patients was calculated as the size necessary to investigate the risk factors (approximately 10 factors) for sarcopenia and muscle mass by univariate and multiple regression analyses. The characteristics of RA patients are presented as means ± standard deviation (SD) for those with a normal distribution or as medians (25th, 75th percentiles) for those not normally distributed. Associations between sarcopenia and MT and between obesity and FT were investigated using Spearman's correlation coefficients on univariate analyses. Receiver operating characteristic (ROC) curve analysis for MT and sarcopenia was performed with MT as a categorical value, and the cut-off value was calculated using the Youden index to maximize the sum of sensitivity and specificity. The correlation between FT and obesity was investigated by a simple linear regression model. The kappa coefficients of sarcopenia and obesity were calculated with the cut-off values of MT and FT. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). P values < 0.05 were considered significant.
3. Results
3.1. Characteristics of RA patients
The details of the clinical data, body composition, and ultrasound parameters of the 84 patients with RA are presented in Table 1. The median age was 66.5 years, and disease duration was 5.3 years. Disease activity was moderate (DAS28-ESR, 3.5). The percentage of methotrexate use was 84.5%, with a mean dose of 8.4 mg/week, and that of glucocorticoid use was 24.2%, with a mean dose of 4.4 mg/day. Body mass index (BMI) was slightly low (21.7 kg/m2), and %BF was intermediate (27.8%). Rectus femoris MT (14.4 mm) was almost the same as vastus intermedius MT (12.8 mm). Weight, height, BMI, ASMI, and basal metabolic rate were higher for men than for women. Muscle function and MT also showed the same tendency. However, body fat percentage and FT were lower in men than in women.
Table 1.
Clinical status, body composition, and ultrasound parameters of all RA patients.
| Variable | RA patients (n = 84) | Men (n = 18) | Women (n = 66) |
|---|---|---|---|
| Age, yr | 66.5 (59, 74) | 68 (65, 74) | 66 (55, 74) |
| Women percentage, % | 78.6 | 0 | 100 |
| Clinical status | |||
| Disease duration, years | 5.3 (1.2, 11.3) | 4.2 (0.6, 6.6) | 6.1 (1.6, 11.3) |
| Stage, I/II/III/IV | 31/25/14/14 | 6/4/4/4 | 25/21/10/10 |
| Class, 1/2/3/4 | 42/39/3/0 | 10/8/0/0 | 32/31/3/0 |
| CRP, mg/dL | 0.11 (0.05, 0.33) | 0.14 (0.06, 0.23) | 0.11 (0.04, 0.34) |
| MMP3, ng/mL | 74.6 (53.6, 124.9) | 109.4 (75.1, 183.7) | 69.3 (50.4, 104.1) |
| ACPA positive, % | 79.8 | 72.2 | 81.8 |
| RF positive, % | 65.5 | 61.1 | 66.7 |
| DAS28-ESR | 3.5 ± 1.0 | 3.3 ± 0.8 | 3.6 ± 1.0 |
| mHAQ | 0.38 (0.13, 0.75) | 0.125 (0, 0.38) | 0.38 (0.13, 0.88) |
| MTX, mg/week, rate (%) | 8.4 ± 2.9 mg, 84.5% | 7.9 ± 3.4 mg, 72.2% | 8.6 ± 2.8 mg, 87.9% |
| GC, mg/day, rate (%) | 4.4 ± 1.7 mg, 24.2% | 6.3 ± 1.4 mg, 22.2% | 3.9 ± 1.6 mg, 24.2% |
| BIA | |||
| Weight, kg | 52.8 ± 9.5 | 59.8 ± 0.9 | 51.0 ± 9.8 |
| Height, m | 1.56 ± 0.08 | 1.64 ± 0.06 | 1.53 ± 0.07 |
| BMI, kg/m2 | 21.7 ± 3.2 | 22.2 ± 2.8 | 21.6 ± 3.8 |
| Body fat percentage, % | 27.8 ± 7.8 | 22.2 ± 6.2 | 28.8 ± 8.5 |
| ASMI, kg/m2 | 6.4 ± 0.8 | 7.1 ± 0.8 | 6.2 ± 0.7 |
| BMR, kcal | 1052.5 (977, 1159.8) | 1213 (1140, 1294.3) | 1026 (957.3, 1098) |
| Muscle function | |||
| Grip strength, kg | 17.8 (14.1, 21.1) | 19.2 (16.2, 24.2) | 16.9 (10.2, 22.2) |
| Gait speed, m/s | 1.1 ± 0.3 | 1.2 ± 0.2 | 1.1 ± 0.4 |
| Ultrasound | |||
| MT, mm | 27.2 ± 5.3 | 27.5 ± 4.3 | 27.1 ± 5.6 |
| Rectus femoris MT, mm | 14.4 ± 2.9 | 15.3 ± 2.5 | 14.1 ± 3.0 |
| Vastus intermedius MT, mm | 12.8 ± 2.9 | 12.2 ± 2.1 | 12.9 ± 3.0 |
| FT, mm | 9.2 ± 3.8 | 6.8 ± 2.0 | 9.9 ± 3.9 |
Data are shown as mean ± standard deviation (SD) or median (25th, 75th percentile).
RA, rheumatoid arthritis; CRP, C-reactive protein; MMP3, matrix metalloproteinase 3; ACPA, anti-citrullinated protein antibody; RF, rheumatoid factor; DAS, Disease Activity Score; ESR, erythrocyte sedimentation rate; mHAQ, modified Health Assessment Questionnaire; MTX, methotrexate; GC, glucocorticoid; BIA, bioelectrical impedance analysis; BMI, body mass index; ASMI, appendicular skeletal mass index; BMR, basal metabolic rate; MT, muscle thickness; FT, fat thickness.
3.2. Comparisons of RA patients with and without sarcopenia and obesity
The results of BIA, muscle function, and ultrasound are presented in Table 2. The percentage of sarcopenia diagnosed by AWGS criteria was 22.6% (n = 19). The percentage of men was 44.4% (n = 8), and that of women was 16.7% (n = 11). The sarcopenia group was significantly older than the no sarcopenia group. The BMI, %BF, and ASMI were significantly lower in the sarcopenia group than in the no sarcopenia group. Muscle functions were relatively lower in the sarcopenia group than in the no sarcopenia group, but there were no significant differences. MT and FT were significantly lower in the sarcopenia group than in the no sarcopenia group.
Table 2.
Muscle mass parameters of RA patients with and without sarcopenia and obesity.
| Variable | Sarcopenia (n = 19) | No sarcopenia (n = 65) | P-value | Obesity (n = 24) | No obesity (n = 60) | P-value |
|---|---|---|---|---|---|---|
| Age, yr | 77 (64.5, 81.5) | 66 (57, 72) | 0.004‡ | 69.5 (63, 73) | 66 (55.5, 74) | 0.300‡ |
| Women percentage, % | 57.9 | 84.6 | 0.023§ | 70.8 | 81.7 | 0.379§ |
| BIA | ||||||
| Weight, kg | 49.4 ± 8.5 | 53.8 ± 9.6 | 0.076† | 61.7 ± 8.7 | 49.3 ± 7.4 | <0.001† |
| BMI, kg/m2 | 19.9 ± 2.5 | 22.2 ± 3.3 | 0.006† | 25.2 ± 3.0 | 20.3 ± 2.1 | <0.001† |
| Body fat, % | 24.0 ± 7.1 | 28.9 ± 7.8 | 0.016† | 35.9 ± 5.7 | 24.5 ± 6.01 | <0.001† |
| ASMI, kg/m2 | 6.0 ± 0.7 | 6.5 ± 0.8 | 0.005† | 6.8 ± 1.0 | 6.3 ± 0.7 | 0.010† |
| Muscle function | ||||||
| Grip strength, kg | 17.5 (12.8, 22.6) | 18.1 (14.2, 20.7) | 0.549‡ | 17.9 (13.4, 22.6) | 17.7 (14.2, 20.7) | 0.902‡ |
| Gait speed, m/s | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.086† | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.226† |
| Ultrasound | ||||||
| MT, mm | 23.8 ± 4.7 | 28.2 ± 5.1 | 0.001† | 28.7 ± 5.9 | 26.6 ± 5.0 | 0.099† |
| Rectus femoris MT, mm | 13.2 ± 3.0 | 14.7 ± 2.8 | 0.038† | 15.2 ± 3.1 | 14.1 ± 2.8 | 0.109† |
| Vastus intermedius MT, mm | 10.6 ± 2.0 | 13.4 ± 2.8 | <0.001† | 13.5 ± 3.1 | 12.5 ± 2.7 | 0.151† |
| FT, mm | 7.4 ± 1.8 | 9.7 ± 4.0 | 0.016† | 11.8 ± 5.2 | 8.2 ± 2.4 | <0.001† |
Data are shown as mean ± standard deviation (SD) or median (25th, 75th percentile). Continuous variables were analyzed using an unpaired Student's t-test† or the Mann–Whitney U test‡. Categorical variables were analyzed using Fisher's exact test§.
RA, rheumatoid arthritis; BIA, bioelectrical impedance analysis; BMI, body mass index; ASMI, appendicular skeletal mass index; MT, muscle thickness; FT, fat thickness.
The percentage of obesity diagnosed by %BF was 28.6% (n = 24). The percentage of men was 38.9% (n = 7), and that of women was 25.8% (n = 17). Weight, BMI, %BF, and ASMI were significantly higher in the obesity group than in the no obesity group. On the other hand, muscle functions of the obesity group were similar to those of the no obesity group. There was no significant difference in MT between the 2 groups. However, FT was significantly higher in the obesity group than in the no obesity group.
3.3. Relationship between MT and sarcopenia
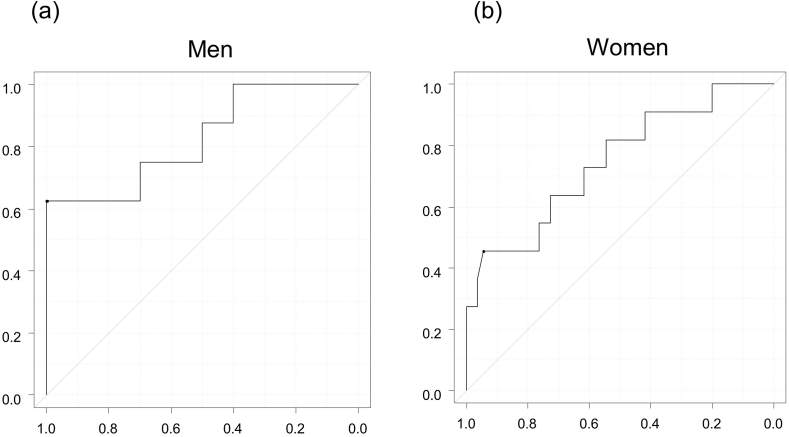
The relationship between MT and sarcopenia was analyzed by sex. MT was significantly negatively correlated with sarcopenia (men: r = −0.56, p = 0.02, women: r = −0.32, P = 0.01). The ROC curve for MT and sarcopenia is presented in Fig. 2. The area under the ROC curve (AUC) for MT was 0.825 (95% CI; 0.620–1.000; P = 0.01) in men; the cut-off value of MT was 24.7 mm (Fig. 2a). The AUC was 0.745 (95% CI; 0.573–0.916; P = 0.03) in women; the cut-off value of MT was 19.7 mm (Fig. 2b). When these cut-off values were used for the diagnosis of sarcopenia, the kappa coefficient was 0.541 (men 0.649, women 0.449). Sensitivity, specificity, and positive and negative predictive values were 52.6%, 95.4%, 76.9%, and 87.3%, respectively.
Fig. 2.
Receiver operating characteristic (ROC) curve analysis of muscle thickness (MT) in relation to sarcopenia. The area under the ROC curve (AUC) for MT is 0.825 in men (a) and 0.745 in women (b).
3.4. Relationship between FT and obesity
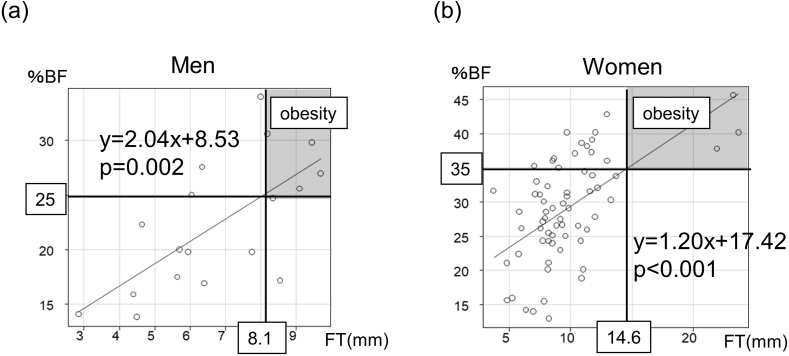
The relationship between FT and obesity was analyzed by sex. FT was significantly positively correlated with %BF (men: r = 0.66, P < 0.01, women: r = 0.62, P < 0.001). A simple linear regression model is presented in Fig. 3. The %BF was estimated by 2.04 × FT + 8.53 in men (Fig. 3a) and by 1.2 × FT + 17.42 in women (Fig. 3b). Using this formula, FT ≥ 8.1 mm in men and FT ≥ 14.6 mm in women indicated obesity. When these cut-off values were used for the diagnosis of obesity, the kappa coefficient was 0.364 (men 0.400, women 0.314). Sensitivity, specificity, and positive and negative predictive values were 96.7%, 33.3%, 78.4%, and 80.0%, respectively.
Fig. 3.
A simple linear regression model between body fat percentage (%BF) and fat thickness (FT). The plot indicates patients, separately for men (a) and women (b). FT ≥ 8.1 mm in men (a) and FT ≥ 14.6 mm (b) in women indicate obesity.
4. Discussion
This study evaluated whether ultrasound examination of the thigh is suitable for screening for sarcopenia and obesity using a cross-sectional analysis of data from a prospective, observational study. The findings indicated that the cut-off value of MT to diagnose sarcopenia was ≤ 24.7 mm in men and ≤ 19.7 mm in women, and the cut-off value of FT to diagnose obesity was ≥ 8.1 mm in men and ≥ 14.6 mm in women.
Ultrasound examinations of healthy individuals were not performed in the present study. Hida et al [19] reported that sarcopenia and thigh muscle thickness were correlated in community-dwelling people; the cut-off values of MT were found to be 36 mm in men and 34 mm in women. The cut-off values in the present study were lower than theirs. The reason may be the different subjects: they investigated healthy community-dwelling people aged ≥ 40 years who participated in annual public health check-ups, whereas the present study investigated outpatients with RA. RA is a chronic inflammatory disease, and the patients’ muscle mass was decreased by joint destruction and deterioration of ADL.
Obesity has been defined using BMI or %BF [21]; %BF was used in the present study, because the average BMI was 21.7 kg/m2, high BMI patients were few, and BMI is calculated by including muscle weight. Therefore, %BF was used in the present study. As for the correlation between FT and obesity, the kappa coefficient was 0.314 in women, which was fair. The reason was that the percentage of obesity was low. If the cut-off value of obesity were 30% in women, the kappa coefficient would be slightly increased, to 0.383 (data not shown).
Whether echogenicity (brightness) of muscle, which reflects the ratio of muscle and fat, reflects muscle quality is controversial. Kawai et al [25] reported that classification of the morphological (echogenicity) and qualitative (thickness) characteristics obtained from ultrasound imaging may be useful for assessing sarcopenia in community-dwelling older adults. However, echogenicity is changed by the setting and model of the ultrasound machine and the direction of the probe. The thickness of muscle is not changed by these conditions and can provide highly reliable data. Berger et al [26] reported that MT was correlated with muscle density (echogenicity) at the rectus femoris. Therefore, the evaluation of MT includes that of muscle quality.
Once patients develop sarcopenia, it is difficult to escape from it, because an increase of muscle mass and recovery of muscle function takes much time. Exercise and nutrition are important to prevent sarcopenia. However, they need to be continued, which requires strong motivation. On the other hand, obesity is one of the risk factors for cardiovascular events [15] and metabolic syndrome [16], and a predictor of worse disease activity in RA [27]. Early diagnosis and intervention are necessary to prevent the development of sarcopenia and obesity. Ultrasound examination of thigh muscle and fat, which is easy and less invasive compared to other examinations [28], is suitable as a screening tool for sarcopenia and obesity and useful as an opportunity for starting exercise and nutritional interventions.
The prevalence of sarcopenia in RA was 28% in the CHIKARA study [9]. Yoshimura et al [29] reported that it was 8.5% in men and 8.0% in women among community-dwelling people in the ROAD study. The prevalence of sarcopenia is higher in RA than in community-dwelling people. Grip strength was not evaluated accurately, because RA patients have finger deformities and joint destruction of the hands. Their gait speed is also slow, because they have destruction of weight-bearing joints (hips, knees, and ankles). Therefore, RA patients have muscle function disabilities. In addition, glucocorticoids, which some RA patients take daily to control their pain, can decrease muscle mass. We have reported that RA patients using GCs at an average dose ≥ 3.25 mg/day over 1 year were at higher risk for developing sarcopenia in the CHIKARA study [30]. It is known that RA induced the metabolic abnormality called “cachexia” [31,32]. It is important to not only control the disease activity, but also prevent sarcopenia.
The present study has some limitations that must be considered. First, disease activity was relatively well controlled, and the percentage of obesity was low. Most patients were active and had mid-level BMIs. Second, the number of patients with RA was small, and there were no healthy controls for comparison. Third, echogenicity, which may reflect muscle quality, was not assessed by ultrasound.
5. Conclusions
The present study showed that ultrasound examination of the anterior thigh was useful to identify sarcopenia and obesity in patients with RA. MT ≤ 24.7 mm in men and ≤ 19.7 mm in women may indicate sarcopenia, and FT ≥ 8.1 mm in men and ≥ 14.6 mm in women may indicate obesity. Measuring MT and FT by US is useful to screen for sarcopenia and obesity in patients with RA.
CRediT author statement
Masahiro Tada: Conceptualization, Formal analysis, Investigation, Writing – Original draft, Writing - Review & editing. Yutaro Yamada: Formal analysis. Koji Mandai: Data Curation. Noriaki Hidaka: Writing –Original draft.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
The authors greatly appreciate the cooperation of the patients with rheumatoid arthritis in the CHIKARA study. This study was supported by a Grant-in-aid for Osteoporosis and Quality of Life 2015 from the Japan Osteoporosis Foundation. ORCID Masahiro Tada: 0000-0003-3161-3929. Yutaro Yamada: 0000-0002-7895-7684. Koji Mandai: 0000-0001-7599-7835. Noriaki Hidaka: 0000-0002-3484-9809.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Schiff M., Keiserman M., Codding C., Songcharoen S., Berman A., Nayiager S. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67:1096–1103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Heijde D., Breedveld F.C., Kavanaugh A., Keystone E.C., Landewe R., Patra K. Disease activity, physical function, and radiographic progression after longterm therapy with adalimumab plus methotrexate: 5-year results of PREMIER. J Rheumatol. 2010;37:2237–2246. doi: 10.3899/jrheum.100208. [DOI] [PubMed] [Google Scholar]
- 3.van der Heijde D., Klareskog L., Rodriguez-Valverde V., Codreanu C., Bolosiu H., Melo-Gomes J. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:1063–1074. doi: 10.1002/art.21655. [DOI] [PubMed] [Google Scholar]
- 4.Peel N.F., Moore D.J., Barrington N.A., Bax D.E., Eastell R. Risk of vertebral fracture and relationship to bone mineral density in steroid treated rheumatoid arthritis. Ann Rheum Dis. 1995;54:801–806. doi: 10.1136/ard.54.10.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Staa T.P., Geusens P., Bijlsma J.W., Leufkens H.G., Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3104–3112. doi: 10.1002/art.22117. [DOI] [PubMed] [Google Scholar]
- 6.Wright N.C., Lisse J.R., Walitt B.T., Eaton C.B., Chen Z., Women's Health Initiative I Arthritis increases the risk for fractures--results from the Women's Health Initiative. J Rheumatol. 2011;38:1680–1688. doi: 10.3899/jrheum.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue Q.L. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tada M., Yamada Y., Mandai K., Hidaka N. Matrix metalloprotease 3 is associated with sarcopenia in rheumatoid arthritis - results from the CHIKARA study. Int J Rheum Dis. 2018;21:1962–1969. doi: 10.1111/1756-185X.13335. [DOI] [PubMed] [Google Scholar]
- 10.Tada M., Yamada Y., Mandai K., Hidaka N. Correlation between frailty and disease activity in patients with rheumatoid arthritis: data from the CHIKARA study. Geriatr Gerontol Int. 2019;19:1220–1225. doi: 10.1111/ggi.13795. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyere O., Cederholm T. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L.K., Liu L.K., Woo J., Assantachai P., Auyeung T.W., Bahyah K.S. Sarcopenia in Asia: consensus report of the asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Bartok C., Schoeller D.A. Estimation of segmental muscle volume by bioelectrical impedance spectroscopy. J Appl Physiol. 2004;96:161–166. doi: 10.1152/japplphysiol.00686.2002. [DOI] [PubMed] [Google Scholar]
- 14.Nijholt W., Scafoglieri A., Jager-Wittenaar H., Hobbelen J.S.M., van der Schans C.P. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle. 2017;8:702–712. doi: 10.1002/jcsm.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubert H.B., Feinleib M., McNamara P.M., Castelli W.P. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 16.Despres J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 17.Welch A.A., Hayhoe R.P.G., Cameron D. The relationships between sarcopenic skeletal muscle loss during ageing and macronutrient metabolism, obesity and onset of diabetes. Proc Nutr Soc. 2019:1–12. doi: 10.1017/S0029665119001150. [DOI] [PubMed] [Google Scholar]
- 18.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., 3rd Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 19.Hida T., Ando K., Kobayashi K., Ito K., Tsushima M., Kobayakawa T. Editors' Choice Ultrasound measurement of thigh muscle thickness for assessment of sarcopenia. Nagoya J Med Sci. 2018;80:519–527. doi: 10.18999/nagjms.80.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallagher D., Heymsfield S.B., Heo M., Jebb S.A., Murgatroyd P.R., Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 21.Okorodudu D.O., Jumean M.F., Montori V.M., Romero-Corral A., Somers V.K., Erwin P.J. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes. 2010;34:791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 22.Haraoui B., Smolen J.S., Aletaha D., Breedveld F.C., Burmester G., Codreanu C. Treating Rheumatoid Arthritis to Target: multinational recommendations assessment questionnaire. Ann Rheum Dis. 2011;70:1999–2002. doi: 10.1136/ard.2011.154179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prevoo M.L., van 't Hof M.A., Kuper H.H., van Leeuwen M.A., van de Putte L.B., van Riel P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 24.Pincus T., Summey J.A., Soraci S.A., Jr., Wallston K.A., Hummon N.P. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 25.Kawai H., Kera T., Hirayama R., Hirano H., Fujiwara Y., Ihara K. Morphological and qualitative characteristics of the quadriceps muscle of community-dwelling older adults based on ultrasound imaging: classification using latent class analysis. Aging Clin Exp Res. 2018;30:283–291. doi: 10.1007/s40520-017-0781-0. [DOI] [PubMed] [Google Scholar]
- 26.Berger J., Bunout D., Barrera G., de la Maza M.P., Henriquez S., Leiva L. Rectus femoris (RF) ultrasound for the assessment of muscle mass in older people. Arch Gerontol Geriatr. 2015;61:33–38. doi: 10.1016/j.archger.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Levitsky A., Brismar K., Hafstrom I., Hambardzumyan K., Lourdudoss C., van Vollenhoven R.F. Obesity is a strong predictor of worse clinical outcomes and treatment responses in early rheumatoid arthritis: results from the SWEFOT trial. RMD Open. 2017;3 doi: 10.1136/rmdopen-2017-000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazzocchi A., Filonzi G., Ponti F., Albisinni U., Guglielmi G., Battista G. Ultrasound: which role in body composition? Eur J Radiol. 2016;85:1469–1480. doi: 10.1016/j.ejrad.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura N., Muraki S., Oka H., Iidaka T., Kodama R., Kawaguchi H. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos Int. 2017;28:189–199. doi: 10.1007/s00198-016-3823-0. [DOI] [PubMed] [Google Scholar]
- 30.Yamada Y., Tada M., Mandai K., Hidaka N., Inui K., Nakamura H. Glucocorticoid use is an independent risk factor for developing sarcopenia in patients with rheumatoid arthritis: from the CHIKARA study. Clin Rheumatol. 2020;39:1757–1764. doi: 10.1007/s10067-020-04929-4. [DOI] [PubMed] [Google Scholar]
- 31.Masuko K. Rheumatoid cachexia revisited: a metabolic co-morbidity in rheumatoid arthritis. Front Nutr. 2014;1:20. doi: 10.3389/fnut.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsmith J., Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol. 2002;85:89–99. doi: 10.1016/s0167-5273(02)00237-1. [DOI] [PubMed] [Google Scholar]