Abstract
Focal ischemic stroke (FIS) is a leading cause of human death. Glial scar formation largely caused by reactive astrogliosis in peri-infarct region (PIR) is the hallmark of FIS. Glial cell-derived neurotrophic factor (GDNF) was originally isolated from a rat glioma cell-line supernatant and is a potent survival neurotrophic factor. Here, using CreERT2–LoxP recombination technology, we generated inducible and astrocyte-specific GDNF conditional knockout (cKO), i.e., GLAST-GDNF−/− cKO mice to investigate the effect of reactive astrocytes (RAs)-derived GDNF on neuronal death, brain damage, oxidative stress and motor function recovery after photothrombosis (PT)-induced FIS. Under non-ischemic conditions, we found that adult GLAST-GDNF−/− cKO mice exhibited significant lower numbers of Brdu+, Ki67+ cells and DCX+ cells in the dentate gyrus (DG) in hippocampus than GDNF floxed (GDNFf/f) control (Ctrl) mice, indicating endogenous astrocytic GDNF can promote adult neurogenesis. Under ischemic conditions, GLAST-GDNF−/− cKO mice had a significant increase in infarct volume, hippocampal damage and FJB+ degenerating neurons after PT as compared with the Ctrl mice. GLAST-GDNF−/− cKO mice also had lower densities of Brdu+ and Ki67+ cells in the PIR and exhibited larger behavioral deficits than the Ctrl mice. Mechanistically, GDNF deficiency in astrocytes increased oxidative stress through the downregulation of glucose-6-phosphate dehydrogenase (G6PD) in RAs. In summary, our study indicates that RAs-derived endogenous GDNF plays important roles in reducing brain damage and promoting brain recovery after FIS through neural regeneration and suggests that promoting anti-oxidant mechanism in RAs is a potential strategy in stroke therapy.
Keywords: Astrocyte, GDNF, neurogenesis, oxidative stress, G5PD, neuronal death, behavioral deficits
INTRODUCTION
Focal ischemic stroke (FIS) is a leading neural disorder that causes brain damage and human disability and death, and thus has a major impact on public health. Despite intense efforts being taken in basic and translational research, the treatment strategies are still limited. Astrocytes are the most numerous and diverse glial cells in CNS, intimately contact with neurons, and fulfill many functions including maintaining brain homeostasis, storing and distributing energy substrates, controlling the development of neural cells and synaptogenesis (Halassa, Fellin, and Haydon;Haydon, 2001; Haydon and Carmignoto, 2006; Kimelberg and Nedergaard, 2010; Zuchero and Barres, 2015). While neuron-centric strategies have not resulted in major breakthroughs in stroke therapy, astrocytes have been recently indicated as a promising target for neural repair in stroke and other brain diseases through either non-cell autonomous or cell autonomous effects on neuronal survival and regeneration (Zhao and Rempe, 2010; Gleichman and Carmichael, 2014; Chouchane and Costa, 2012; Kimelberg and Nedergaard, 2010; Escartin and Bonvento, 2008; Ding, 2014; Choudhury and Ding, 2016; Lobsiger and Cleveland, 2007; Jolly et al., 2011; Li, Xie, Zhang, Yu, Zhang, and Ding, 2015; Maragakis and Rothstein, 2006; Pecho-Vrieseling et al., 2014). Following the onset of a FIS, the brain experiences a series of spatiotemporally dependent changes in pathological processes. In the ischemic core (IC), neurons die rapidly from the onset of FIS due to severe energy failure and the ensuing breakdown of ion homeostasis. In the peri-infarct region (PIR), the region that surrounds the IC, the brain tissue is hypoperfused with collateral blood flow and preserves partial energy metabolism. Thus, the primary goal for stroke therapy is to salvage the PIR tissue after FIS. Surviving neurons in the PIR exhibit active structural and functional synaptic rewiring and remapping during subacute phase (days after FIS), which contributes to long-term neural regeneration and functional recovery (Brown, et al., 2009; Clarkson et al., 2013; Murphy and Corbett, 2009; Mostany et al., 2008; Winship & Murphy, 2008). Another prominent feature of FIS is reactive astrogliosis and glial scar formation in the PIR. Astrocytes in the PIR exhibit dynamic changes in morphology, proliferation and gene expression during the subacute phase (Barreto, Sun, Xu, and Giffard, 2011; Li et al., 2014; Choudhury and Ding), e.g., the upregulation of GFAP in astrocytes is a hallmark of reactive astrogliosis after FIS, and therefore these astrocytes are called reactive astrocytes (RAs). After a prolonged time following FIS, the morphology of RAs remains stable, the proliferation of RAs ceases, and glial scar is formed (Barreto, Sun, Xu, and Giffard, 2011; Li et al., 2014; Ding, 2014;Choudhury and Ding, 2016; Burda and Sofroniew, 2014; Voskuhl et al., 2009). Reactive astrogliosis and glial scar formation eventually cause substantial tissue remodeling and permanent structural changes in the PIR. As RAs exhibit dynamic changes after a FIS, genetic manipulations of these resident cells under permissive conditions (e.g., before glial scar formation) to stimulate neural regeneration could be a novel approach for stroke therapy.
GDNF is a member of transforming growth factor (TGF)-β (Airaksinen and Saarma, 2002) and also a potent neurotrophic factor that promotes neuronal survival, neuroprogenitor differentiation, and synapse formation (Nakajima et al., 2001; Ledda, Paratcha, Sandoval-Guzman, and Ibanez, 2007; Chen, Ai, Slevin, Maley, and Gash, 2005; Airaksinen and Saarma, 2002; Pascual et al., 2008; Uesaka, Nagashimada, and Enomoto, 2013). GDNF is originally isolated from a rat glioma cell-line supernatant as a trophic factor for embryonic midbrain dopaminergic neurons, and was later found to have pronounced effects on other neuronal subpopulation (Lin, Doherty, Lile, Bektesh, and Collins, 1993). GDNF also protects dopaminergic and spinal motor neurons, and thus it has received considerable attention as a drug candidate for the treatment of Parkison’s Disease (PD) and other neurodegenerative diseases (Luz, Mohr, and Fibiger, 2016; Kirik, Georgievska, and Bjorklund, 2004). The effects of GDNF on brain protection in stroke were also evaluated owning to the findings that the production of GDNF and expression of its receptors RET and GFRα-1 were upregulated (Abe and Hayashi, 1997; Kitagawa et al., 1999; Wei, Wu, and Cao, 2000; Miyazaki, Nagashima, Okuma, and Nomura, 2001; Arvidsson et al., 2001; Kuric, Wieloch, and Ruscher, 2013). Administration of recombinant GDNF protects against brain damage after middle cerebral artery occlusion (MCAo)(Wang et al., 1997; Kobayashi et al., 2006; Kitagawa et al., 1998; Jin et al., 2003; Horita et al., 2006; Shang et al., 2010). Genetic strategies such as viral transduction to increase local production of GDNF were also tested for stroke therapy, 2003; Hermann et al., 2001). These studies have demonstrated that GDNF is neuronal and brain protective, and is a promising candidate for stroke therapy. Although GDNF is originally isolated from a rat glioma cell-line supernatant (Airaksinen and Saarma, 2002) and can be released from cultured astrocytes (Yamagata et al., 2007; Rocha et al, 2012; Yan et al., 2011; Chen et al., 2015) probably because they are more akin to RAs (Foo et al., 2011), GDNF is mostly confined in neurons and absent from or expressed little in astrocytes in the normal brain (Miyazaki, Nagashima, Okuma, and Nomura, 2001; Hidalgo-Figueroa et al., 2012; Kuric, Wieloch, and Ruscher, 2013). Consistently, RNA-seq study also showed that mature mouse neurons express much higher GDNF mRNA than mature mouse astrocytes (Zhang et al., 2014). However, studies showed that GDNF is upregulated in RAs after FIS (Abe and Hayashi, 1997; Kitagawa et al., 1999; Wei, Wu, and Cao, 2000; Miyazaki, Nagashima, Okuma, and Nomura, 2001; Arvidsson et al., 2001), suggesting that RAs may serve as the main source of GDNF to stimulate neural regeneration during the post-ischemic time. Nevertheless, whether and how astrocyte-specific deletion or overexpression of GDNF using molecular genetic approaches affect FIS has not been reported.
Here we generated astrocyte-specific, inducible and conditional GDNF knockout (cKO) mice, i.e., GLAST-GDNF−/− cKO mice by crossing floxed GDNF (GDNFf/f) mice with GLAST-CreERT2 driver line. The aim of this work is to study the effect of endogenous astrocytic GDNF on brain infarction, neurodegeneration, reactive astrogliosis, oxidative stress and behavioral deficits after photothrombosis (PT)-induced FIS. Giving that GLAST is also expressed in neural stem cells, we also studied the effect of GDNF deletion in astrocytes on adult neurogenesis in dentate gyrus (DG) in hippocampus. Our findings indicate that RAs-derived GDNF plays a critical role in protecting against neuronal degeneration and brain damage, stimulating reactive astrogliosis, promoting anti-oxidative stress mechanism and improving long-term stroke outcomes after FIS.
MATERIALS AND METHODS
Animals
Adult male and female wild type (WT) and cKO mice with C57BL/6J background aged 8–10 weeks were used in this study. Mice were maintained on a 12 h light:12 h dark cycle (lights on 7 am-7 pm) under pathogen-free conditions in the AAALAC-accredited animal facility at the University of Missouri according to institutional guidelines. All experimental procedures were performed according to the NIH Guide for the Care and Use of Laboratory Animals and approved by the University of Missouri Animal Care Quality Assurance Committee (ACQAC). Adult mice of both male and female mice were used in the current study.
Generation of GDNF cKO Mice
To generate astrocyte-specific GDNF cKO mice, we used CreERT2–LoxP recombination technology. GDNFf/f mice (Shneider, Brown, Smith, Pickel, and Alvarez, 2009) (Stock No. 014097, The Jackson Laboratory, Bar Harbor, ME) were crossed with GLAST-CreERT2 driver mice expressing inducible form of Cre recombinase under the control of GLAST promoter (Mori et al., 2006) (Stock No. 012586, The Jackson Laboratory) to obtain GDNF+/f:GLAST-CreERT2 bitransgenic mice. GDNF+/f:GLAST-CreERT2 mice were further bred with GDNFf/f mice to obtain GDNFf/f:GLAST-CreERT2 homozygous bitransgenic mice. Genotypes were confirmed by PCR amplification of their DNA from tail snips (Li et al., 2015; Wang et al., 2017). GDNFf/f mice were used as WT or control (Ctrl) mice for respective bitransgenic mice. To delete GDNF in GDNFf/f:GLAST-CreERT2 mice, tamoxifen (TAM) (100 mg/kg in sunflower seed oil, Cat. No. T5648, Sigma, Saint Louis, MO) was administered for 5 consecutive days by oral gavage at the age of 6 weeks (Wang et al., 2017). These mice were ready to use 2 weeks later. We designate these TAM-treated GDNFf/f:GLAST-CreERT2 mice as GLAST-GDNF−/− cKO mice. Sunflower seed oil was administered in GDNFf/f mice for control experiments. Since GLAST promoter is very active in developmental stage, inducible deletion of GDNF in adult mice by Cre recombination will bypass the developmental compensation.
Photothrombosis (PT)-induced ischemia model
PT was induced as described in our previous studies (Li et al., 2015; Li, Zhang, Sun, and Ding, 2013; Zhang et al., 2010; Ding et. al., 2009; Li et al., 2014). Briefly, mice were anesthetized by ketamine and xylazine (130 mg and 10 mg/kg body weight). Rose Bengal (30 mg/kg dissolved in saline) (Cat. No. 330000, Sigma) was injected through the tail vein. To induce a FIS, an area of 1.5 mm diameter was focally illuminated 3 min later on the intact skull without skin at the center of −0.8 mm from the bregma and 2.0 mm lateral to the midline (motor cortex) for 2 min with a green light (540–580 nm) through a ×10 objective. The light source was an X-cite 120 PC metal halide lamp (Excelitas Technologies, Waltham, MA).
Transcardial perfusion, infarct volume and hippocampal damage measurements, and FJB staining
Transcardial perfusion, infarct volume measurement and FJB staining were described as in our previous studies (Li, Zhang, Sun, and Ding, 2013; Li et al., 2015; Zhang et al., 2010; Wang et al. 2017). Briefly, mice were transcardially perfused with phosphate buffer saline (PBS), followed by ice-cold 4% paraformaldehyde (PFA) in PBS (pH 7.4). After perfusion, the brain was removed and post-fixed in 4% PFA in PBS at 4 °C overnight, and was then transferred to 30% sucrose for 2–3 days until sink. Coronal sections of the brain (30 μm) were cut using a cryostat, collected serially on pre-gelatin coated glass slides, and stored at −20 °C until use. For infarct volume measurement, brain sections were stained by 0.25% cresyl violet (Nissl) and the areas of cerebral infarction were delineated and quantified using ImageJ software (NIH). The infarct volume was calculated as infarct area times the thickness of brain section as in our previous studies. For hippocampal damage measurement, the damaged area of hippocampus in ipsilateral hemisphere and the whole area of hippocampus in the contralateral hemisphere in Nissl stained sections were delineated and calculated. The ratio of the total damaged hippocampal area to the whole hippocampal area was defined as the percentage of hippocampal damage. For detecting degenerating neurons, we used Fluoro-Jade B (FJB) staining (Li et al., 2015; Zhang et al., 2010). The brain sections on glass slides were washed with ddH2O and immersed in 0.06% potassium permanganate for twenty minutes, and then immersed into 0.0004% FJB in 0.1% acetic acid solution for 45 min in dark.
5-Bromo-2-deoxyuridine (Brdu) injection
To evaluate adult neurogenesis, Brdu (Cat. No. B9285, Sigma) was administered through intraperitoneal (IP) injection with a dose of 50 mg/kg in saline for 6 consecutive days (Li, Zhang, Sun, and Ding, 2013; Li et al., 2014). Mice were anesthetized and transcardially perfused 1 day or 28 days following the last injection for evaluating short and long-term effect of astrocytic GDNF on neurogenesis.
In vivo labeling of reactive oxygen species (ROS) by dihydroethidium (DHE)
To evaluate oxidative stress, we administered DHE through IP injection to label ROS. Specifically, DHE (25 mg/kg body weight, Cat. No. 37291, Sigma) was injected for two times within 30 min at 6 h after PT and sacrificed 18 h later. Brain sections were cut and counter-stained with Dapi, and imaged immediately using fluorescent microscope.
Immunostaining
The procedures of immunostaining were described in our previous studies (Wang et al., 2017; Li, Zhang, Sun, and Ding, 2013; Li, Zhang et al., 2014). Brain sections were sequentially incubated overnight at 4°C with primary antibodies, then incubated with an Alexa 488-conjugated donkey anti-rat IgG (1:200; 712-548-153, Jackson ImmunoResearch Laboratories, West Grove, PA), a Alexa 488-conjugated donkey-anti-mouse IgG (1:200; Life Technologies, CA, USA), an Alexa 594-conjugated donkey anti-rabbit IgG (1:200; 711-585-152, Jackson ImmunoResearch Laboratories) for 4 hr in the dark at room temperature. The brain sections were subjected to fluorescence detection using a Nikon FN1 epi-fluorescence microscopy equipped with a CoolSNAP-EZ CCD-camera or an Olympus confocal microscope and analyzed by MetaMorph software (Molecular Devices, USA). The primary antibodies include a rabbit anti-GDNF polyclonal antibody (1:200; SC-328, Santa Cruz Biotechnology, Santa Cruz, CA), a rabbit anti-Ki67 (1:600, AB9260, Sigma), a rabbit anti-doublecortin (DCX) (1:1000; Ab18723, Abcam, MA. RRID:AB_732011), a mouse anti-S100β monoclonal antibody (1:500; S2532, Sigma. RRID:AB_477499), a mouse anti-GFAP (1:800; G3893, Sigma. RRID:AB_477010), a rat anti-Brdu (1:400; Ab6326, Abcam. RRID:AB_305426), and a rabbit G6PD (1:400; NB100-236, Novus Biologocals, Centennial, CO. RRID:AB_2107663).
Cell counting
The numbers of FJB+ cells and immunostained cells in the PIR and hippocampus were counted using the MetaMorph Imaging software as in our previous studies (Zhang et al., 2010; Xie, Wang, Sun, and Ding, 2010). Cells were counted if they contained a whole-cell body and were presented as per mm2 or per slice. The data were averaged and expressed as mean±s.e.m.
Western blot analysis
Western blot (WB) was performed to analyze protein levels as described in our previous studies (Li eat al., 2015;Li, Zhang, Sun, and Ding, 2013; Wang et al., 2017; Zhang et al., 2010). Briefly, total protein was extracted from freshly-harvested brain cortex or hippocampus using a lysis buffer (pH 8.2) plus protease inhibitor phenylmethylsulfonyl fluoride (PMSF) (Cat. 36978, ThermoFisher Scientific, Waltham, MA), and phosphatase inhibitor cocktails (Cat. No. P8340, Sigma). The homogenized tissue was centrifuged at 12,000× g for 10 min at 4 °C. The supernatant is the total cell lysate. The protein concentration of cell lysate was determined with a BCA protein assay kit (Cat. 23227, Thermoscientific). Equivalent amounts of protein from each sample were diluted with Laemmli buffer, boiled for 5 min, subjected to electrophoresis in 10% SDS-polyacrylamide gels at 100 mV and subsequently transferred to PVDF membranes. Membranes were blocked for 1 h with 5% BSA in Tris-buffered saline containing 0.1% Tween 20 (TBST) and were incubated in TBST overnight at 4 °C with first antibodies including mouse anti-GDNF (1:1000; bs-1024R, Bioss Antibodies, Boston, MA. RRID:AB_10883645), mouse anti-synaptophysin (synapt) (1:1000; S-5768, Sigma. RRID:AB_477523), mouse anti- vesicular glutamate transporter 1 (vGlut1) (1:1000; NBP2-59329, Novus Biologicals, LLC, Centennial, CO, USA), rabbit anti-NMDAR2A (1:1000; NB300-105, Novus Biologicals, LLC. RRID:AB_10001400), rabbit anti-NMDAR2B (1:1000; NB300-106, Novus Biologicals, LLC. RRID:AB_10000537), mouse anti-GluA1 (1:1000; NBP2-22399, Novus Biologicals, LLC), rabbit anti-GluA2 (1:1000; NBP2-75510, Novus Biologicals, LLC), mouse anti- postsynaptic density protein 95 (PSD95) (1:1000; NB300-556 Biologicals, LLC. RRID:AB_2092366), and mouse anti-β-actin (1:10000; sc-47778, Santa Cruz. RRID:AB_2714189) antibodies. The membranes were then incubated with second antibodies including goat HRP (Horseradish peroxidase)-conjugated anti-rabbit IgG (1:5000; A0545, Sigma. RRID:AB_25896) or rabbit HRP-conjugated anti-mouse IgG (1:5000; A9044, Sigma. RRID:AB_258431) diluted in 5% (w/v) non-fat dry milk in TBS-T for 1 hour at room temperature. The protein bands in the membrane were detected by Gel Documentation Imaging System (Bio-Rad, Hercules, CA).
Behavioral tests
Open field test and motor behavioral tests including hanging wire, cylinder, rotarod and grip force tests were conducted according to our previous studies (Li et al., 2014; Li et al., 2015; Wang et al., 2017). For each type of motor behavior tests, the pre-training was conducted twice before pre-recording of behavior tests. For open field test, after about 30 min habituation in the open field test room, the mouse was placed in the center of the open field apparatus consists of a clear, open Plexiglas box (46.6 cm×38.5 cm×25.6 cm) with an overhead camera to record horizontal movements over a 10-min period by ANY-maze software (Stoelting, IL). Parameters included total travel distance, corner distance, total immobile time, the ratio of corner travel distance to total travel distance and time spent in the corner were quantified by ANY-maze software.
Data analysis and statistics
Data were expressed as means ± SEM. All experiments were performed at least three times and the exact numbers of experiment or replicate are indicated in the figure legends. Statistical comparisons were made by t-test for two groups (Microsoft Excel software) and one-way ANOVA test for multiple groups followed by Bonferroni’s post-hoc test (Origin Pro software, OriginLab Corporation, Northampton, MA).
RESULTS
GDNF is highly upregulated in RAs after PT-induced FIS
To study GDNF expression after FIS, we conducted WB analysis to determine GDNF levels in normal mouse brains and ipsilateral and contralateral hemispheres of ischemic mouse brains at different times after PT. We found that PT caused a dramatic upregulation of GDNF in the ipsilateral hemisphere as compared to normal brain and contralateral hemisphere of ischemic brain (Figure 1A–B). The GDNF levels in the ipsilateral hemisphere reached a peak value about half to one day post PT but maintained at a high level for a prolonged time. Interestingly, GDNF levels in the contralateral hemisphere also exhibit significant increases as compared with normal brains. Consistently, immunostaining data show that GDNF was highly upregulated in many RAs in the PIR close to ischemic core (IC) (e.g., regions A1 and A2), but less in the region distant from IC (e.g., region A3) (Figure 1C). Our data indicate that GDNF is an ischemic stress-responsive gene. Giving the fact that GDNF is a potent neurotrophic factor, our data also suggest that transient increase in GDNF may serve to mediate endogenous neuronal/brain protection, neural regeneration and spontaneous brain recovery after FIS. Since GDNF is also expressed in RAs, RAs-derived GDNF could play an important role in these processes.
Figure 1. GDNF is dramatically upregulated after PT.
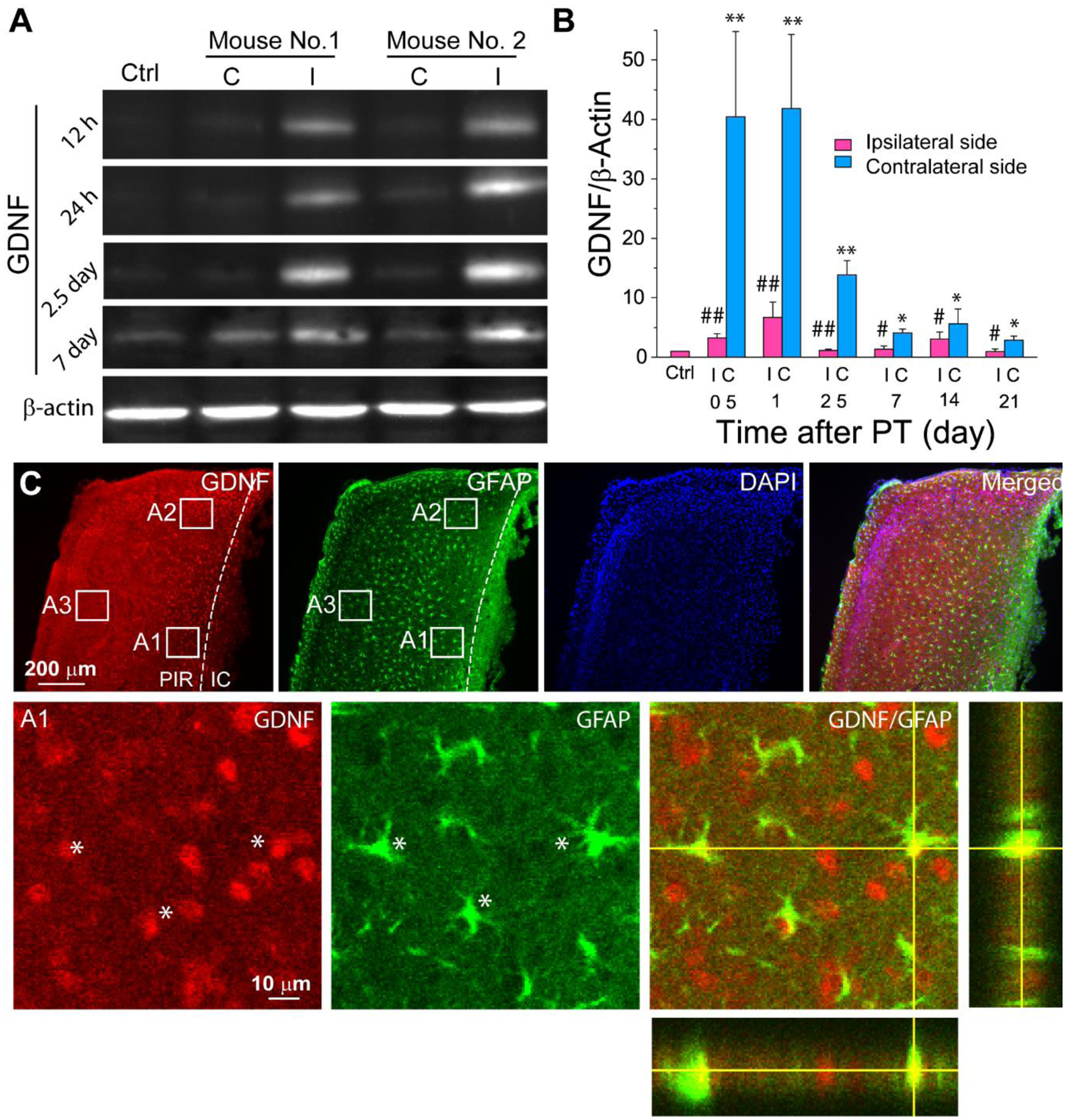
A) WB images of GDNF at different times after PT. B) WB analysis of GDNF showing dramatic increase after PT. Data were analyzed as fold change of GDNF/β-actin compared with normal control mice. N=3–5 mice for each time point. *, #, p<0.05, **, ##, p<0.005 compared with the control group, ANOVA test. C) Confocal images of immunostaining show the colocalization of GDNF with GFAP+ RAs (*) in the PIR 4 days after PT (2015). The bottom panels are the high resolution images of the boxed region A1 and the orthogonal analysis. C-contralateral hemisphere; I-ipsilateral hemisphere; IC-ischemic core; PIR-peri-infarct region.
Generation and characterization of GLAST-GDNF−/− cKO mice
To investigate the role of astrocyte-derived GDNF in FIS, we generated astrocyte-specific, inducible GDNF cKO mice using CreERT2–LoxP recombination technology. Because GLAST-CreERT2-mediated recombination was widely used to express transgenes or delete genes in astrocytes in vivo (Regan et al. 2007; Paukert et al., 2014; Bardehle et al., 2013; Jensen et al., 2016), we crossed GDNFf/f mice with GLAST-CreERT2 driver mice to obtain GLAST-CreERT2:GDNFf/f homozygous bitransgenic mice (Figure 2A). Genotype was confirmed by PCR and deletion of GDNF was achieved by TAM oral gavage for 5 consecutive days to obtain GLAST-GDNF−/− cKO mice (Figure 2B–C). GLAST-GDNF−/− cKO mice and GDNFf/f control mice display no difference in body weight for both male and female mice (Figure 2D), and based on Nissl staining, they do not exhibit any gross anatomic abnormalities of the whole brain and cytoarchitecture of cortex and hippocampus (Figure 2E), and brain weight difference (Figure 2F–G). WB analysis indicates that GLAST-GDNF−/− cKO mice exhibited reduced GDNF levels in both cortex and hippocampus as compared with the control mice (0.839±0.032 vs 1.00±0.043 in the cortex, p=0.01, and 0.780±0.127 vs 1.00± 0.195 in the hippocampus, p=0.36, t-test. N=7 mice for each group) (Fig 2H–I). A small reduction of GDNF in the cKO mice suggests low GDNF levels in astrocytes in the normal brains. To determine whether GDNF deletion potentially affects neuronal function, we examined the expression levels of several important presynaptic and postsynaptic proteins (Figure 2J–K). We found presynaptic proteins synaptophysin (synapt) and vesicular glutamate transporter 1 (vGlut1) levels are not different in the cKO mice compared with the control mice. For post-synaptic proteins, we found that Glu2A and Glu2B subunits of NMDAR, GluA1 subunit of AMPAR, and PSD95 are not changed but GluA2 subunit of AMPAR is significantly increased after GDNF deletion in astrocytes. Overall, these data indicate that the deletion of GDNF in astrocytes has no significant effect on synaptic function under normal conditions.
Figure 2. Generation and characterization of GLAST-GDNF−/− cKO mice.
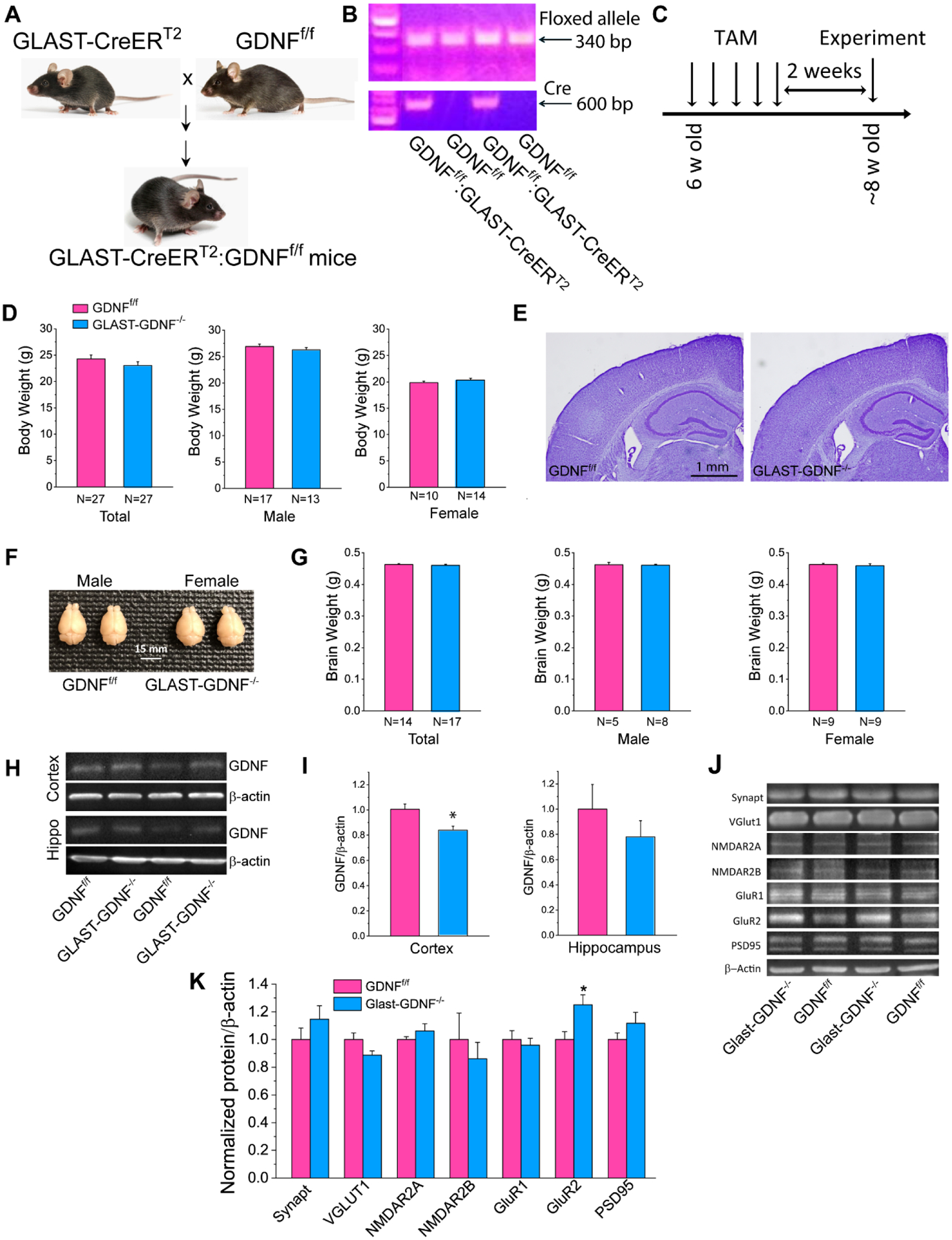
A) Breeding scheme to obtain tamoxifen (TAM) inducible and astrocyte-specific GLAST-GDNF−/− cKO mice. B) Gel images of genotyping. C) Experimental timeline. D) Average body weights of total, male and female of GDNFf/f and GLAST-GDNF−/− cKO mice. E) Nissl staining of brain sections showing cortical and hippocampal structure. F-G) Photographs of whole brain (F) and average brain weight (G). H-I) WB analysis of GDNF levels in the cortex and hippocampus of GDNFf/f and GLAST-GDNF−/− cKO mice using mouse anti-GDNF antibody. Summary data were obtained from the averaged values of N=5 mice for each group. J-K) WB analysis of pre- and post-synaptic proteins in the cortex (and hippocampus). Summary data were obtained from the averaged values of N=5 mice for each group.
Next, we examined GLAST-CreERT2 mediated gene recombination pattern. We crossed GLAST-CreERT2 mice with floxed GCaMP5-IRES-tdTomato mice (Gee et al., 2014) (i.e., GLAST-CreERT2:GCaMP5-IRES-tdTomatof/f) and evaluated the efficiency of GLAST-CreERT2-mediated gene recombination by examining tdTomao expression following the TAM injection. The recombined tdTomato+ astrocytes can be directly visualized by fluorescent imaging and accounted for ~25% of total s100β+ astrocytes when mice were sacrificed 2 weeks after TAM injection (Figure S1A-B). It is also noticeable that tdTomato+ cells are densely packed in subgranular zone (SGZ), and the morphology of these tdTomato+ cells is different from those tdTomato+ astrocytes in the other regions (Figure S1C-D), consistent with the report that GLAST promoter is active in neural stem/progenitor cells (NSPCs) in SGZ (Regan et al., 2007).
GLAST-CreERT2-mediated conditional GDNF deletion reduces adult neurogenesis and NSPC survival in DG under normal conditions
GDNF is a potent neurotrophic factor that promotes neuroprogenitor differentiation. Since GLAST-CreERT2-mediated gene recombination also occurs in SGZ (Figure S1), thus, we initially we examined the effect of GLAST-CreERT2 mediated GDNF deletion on adult neurogenesis in DG using Brdu labeling and Ki67 staining. We administered Brdu in both GDNFf/f mice and GLAST-GDNF−/− cKO mice by IP injection for 6 consecutive days. The mice were then sacrificed one day after the last injection and brains were fixed for immunostaining to examine neurogenesis in DG of hippocampus (Figure 3A–B). Our data show that GLAST-GDNF−/− cKO mice exhibited a significant reduction in Brdu+ and Ki67+ proliferating cells in SGZ of DG as compared with GDNFf/f control mice (Figure 3C–F). These cKO mice also exhibited shorter processes in and a lower density of DCX+ neuroblasts in DG than the control mice (Figure 3G–H).
Figure 3. Deletion of astrocytic GDNF reduces adult neurogenesis in DG under normal conditions.
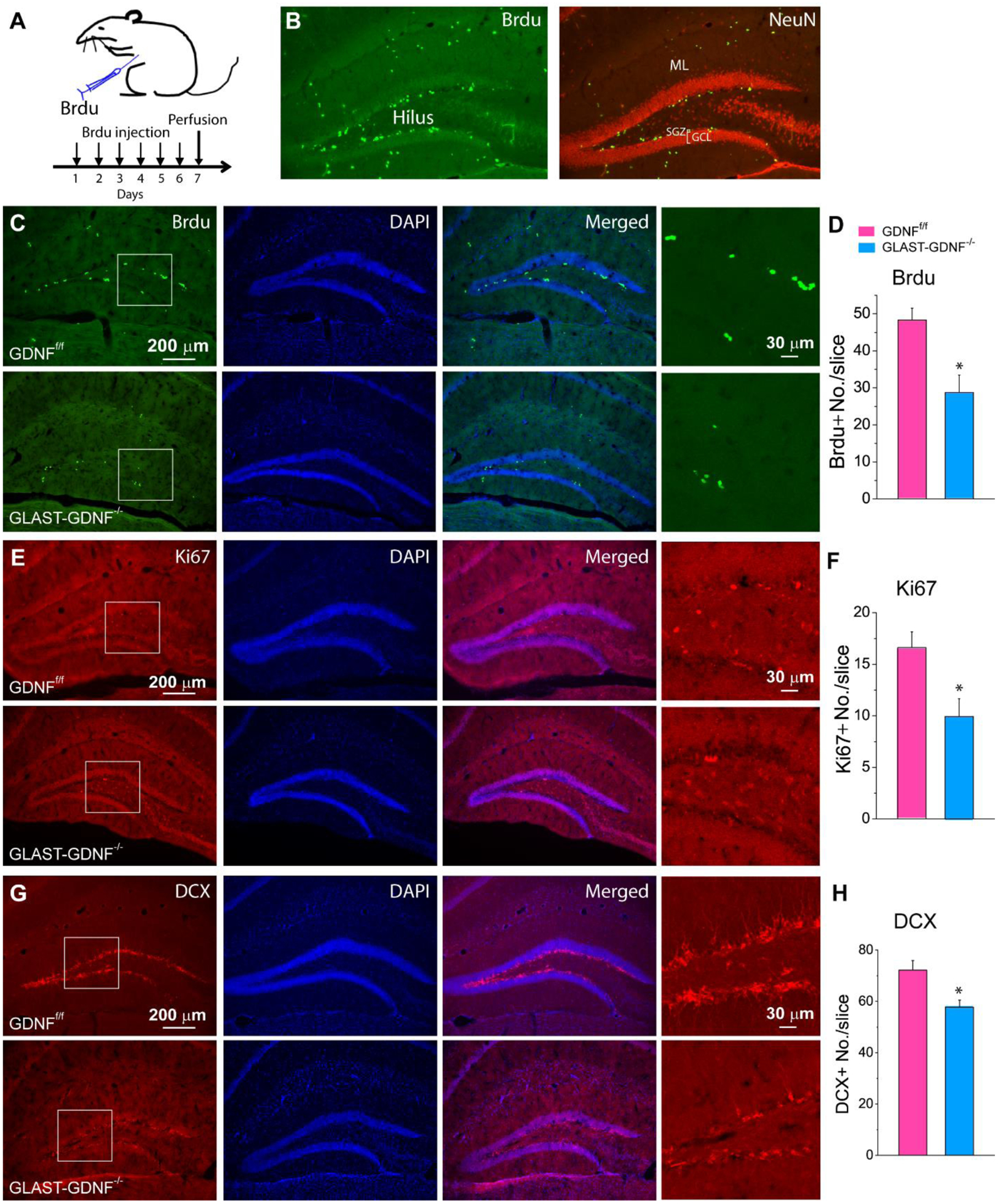
A) Experimental protocol for Brdu injection. B) Immunostaining of Brdu and NeuN for the illustration of adult neurogenesis in the dentate gyrus (DG) of hippocampus. SGZ-subgranular zone; GCL-granular cell layer; ML-molecular layer. C, E, G) Fluorescent images of Brdu/Dapi (C), Ki67/Dapi (E) and DCX/Dapi double staining in DG of GDNFf/f and GLAST-GDNF−/− cKO mice. D, F, H) Summary data of Brdu+ cells (D), Ki67+ cells (F) and DCX+ neuroblasts (H). Four-weeks old GLAST-CreERT2:GDNFf/f mice were injected with TAM (100 mg/Kg) and sacrificed two weeks after last injection (also see Fig 2). Data were averaged from N=4 mice for each genotype and 2 slices of each mouse. *p<0.05, t-test.
Neuron-born neurons in DG die with several days. To determine if the reduction in the number of newborn neurons detected in GLAST-GDNF−/− cKO mice is caused by a reduced cell survival, we traced and counted the number of surviving BrdU+ cells in in SGZ and GCL of GDNFf/f and GLAST-GDNF−/− cKO mice 28 days after the last BrdU injection (Figure 4A). The progeny of Brdu+ labeled neural stem cells will migrate and differentiate under this experimental condition. Our data show that the numbers of Brdu+ in SGZ and GCL in GLAST-GDNF−/− ckO mice were reduced significantly as compared with the control mice (Figure 4B–C). Sox2 is a type 1 neural stem cell (NSC) marker and NeuroD1 is a type 3 intermediate neuronal progenitor (INP) marker (Hodge, Kahoud, and Hevner, 2012). There is also a significant reduction in Brdu+Sox2+ survival progenitor cells, but no significant reduction in Brdu+NeuroD1 survival progenitor cells (Figure 4D–E). Nevertheless, the numbers of Brdu+Sox2+ and Brdu+NeuroD1+ cells are very small. These results suggest that GLAST-CreERT2 mediated GDNF deletion does not cause a significant effect of long-term neuronal stem/progenitor survival.
Figure 4. GLAST-mediated GDNF deletion reduces neuronal stem cell and progenitor cell survival in DG under normal conditions.
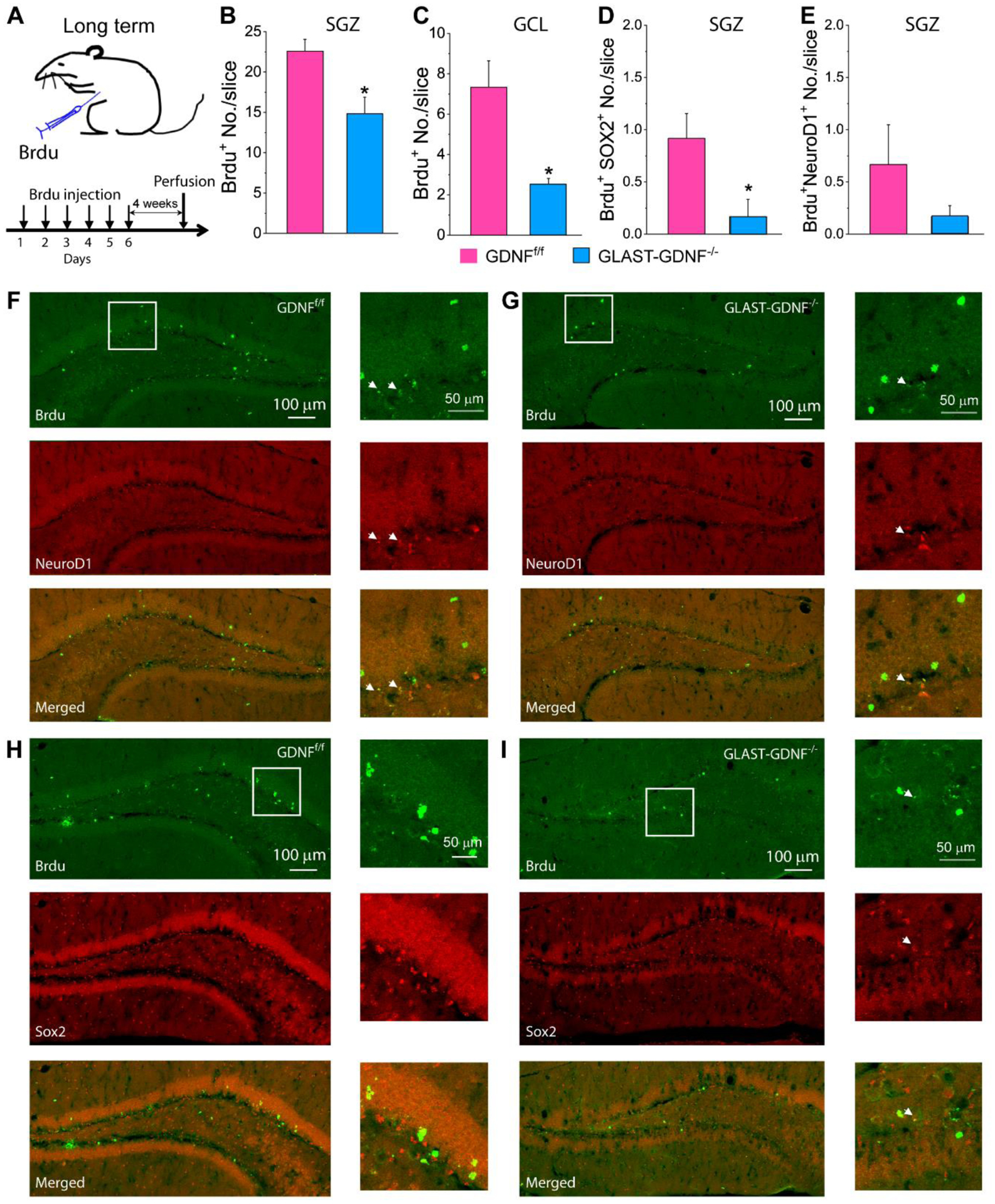
A) Brdu injection and experimental protocol. B-E) Summary results of immunostaining for Brdu+ cells in the SGZ (B) and GCL (C), Brdu+Sox2+ cells (D) and Brdu+NeurD1+ cells (E) in SGZ 4 weeks after Brdu injection. Data in B-E) were averaged from N=6 mice for each genotype and 2 slices for each mouse. *p<0.05, t-test. F-I) Confocal images of Brdu+ and NeuroD1 (F-G), and Brdu and Sox2 double staining (H-I) of GDNFf/f mice (F) and GLAST-GDNF−/− cKO mice (G) 28 days after TAM administration. Images in the right panels are the high-resolution images of the boxed regions in the left panels. Arrowheads point to the cells with colocalization of indicated markers.
The aforementioned data demonstrate that GLAST-promoter mediated GDNF expression can stimulate adult neurogenesis and NSPC proliferation in DG in the healthy brain, which give insights into the role of RAs derived GDNF in reactive astrogliosis and neuronal survival after ischemia.
Increased Cre recombinase (Cre) expression in RAs in GLAST-CreERT2 mice after PT
To further gain insights into how FIS affects Cre activity controlled by GLAST promoter, GLAST-CreERT2 mice were subject to PT and sacrificed two days later. We then conducted immunostaining using anti-Cre antibody. Compared with non-ischemic hemisphere (Figure S2A-B), many cells with high Cre expression levels appeared in the PIR (Figure S2C-D); in addition, double staining of GFAP and Cre indicated that almost 100% of Cre+ cells are GFAP+ RAs with high levels of Cre expression (Figure S2D). These data indicate that GLAST promoter is highly activated in RAs after PT and that FIS can induce a high gene recombination in RAs. These data also suggest that the GLAST-GDNF−/− cKO mice can be used as a loss-of-function tool to delete GDNF in astrocytes under normal conditions and RAs after PT for studying its effect on ischemic brain damage and long-term stroke outcomes.
GLAST-GDNF−/− cKO mice exhibit increased brain and hippocampal damage and neuronal degeneration after PT
Next, we evaluated brain infarct volume and neuronal death in GDNFf/f control mice and GLAST-GDNF−/− cKO mice after PT to determine the effect of astrocytic GDNF on brain damage and neuronal death after PT. We induced PT 2 weeks after TAM injection and sacrificed mice at different times. GLAST-GDNF−/− cKO mice exhibited a significantly larger brain infarct volume at 2, 4 and 14 days after PT than the GDNFf/f control mice (Figure 5A–C). Similarly, hippocampal damage is also significantly increased in the cKO mice 2 and 4 days after PT (Figure 5D). Moreover, GLAST-GDNF−/− cKO mice exhibited a higher density of FJB+ degenerating neuron in the PIR than the control mice 2 days after PT (Figure 5E–F). Using confocal imaging, we also confirmed that GDNF were absent in many RAs from GLAST-GDNF−/− cKO mice as compared with the control mice (Figure 5G–H). Consistently, based on WB analysis, GDNF levels in the ischemic side of the cKO mice were lower than the control mice (Figure 5I–J). These data demonstrate that astrocytic GDNF plays a protective role against neuronal death and brain damage after FIS.
Figure 5. Deletion of GDNF in astrocyte increases ischemic infarction and neuronal degeneration after PT.
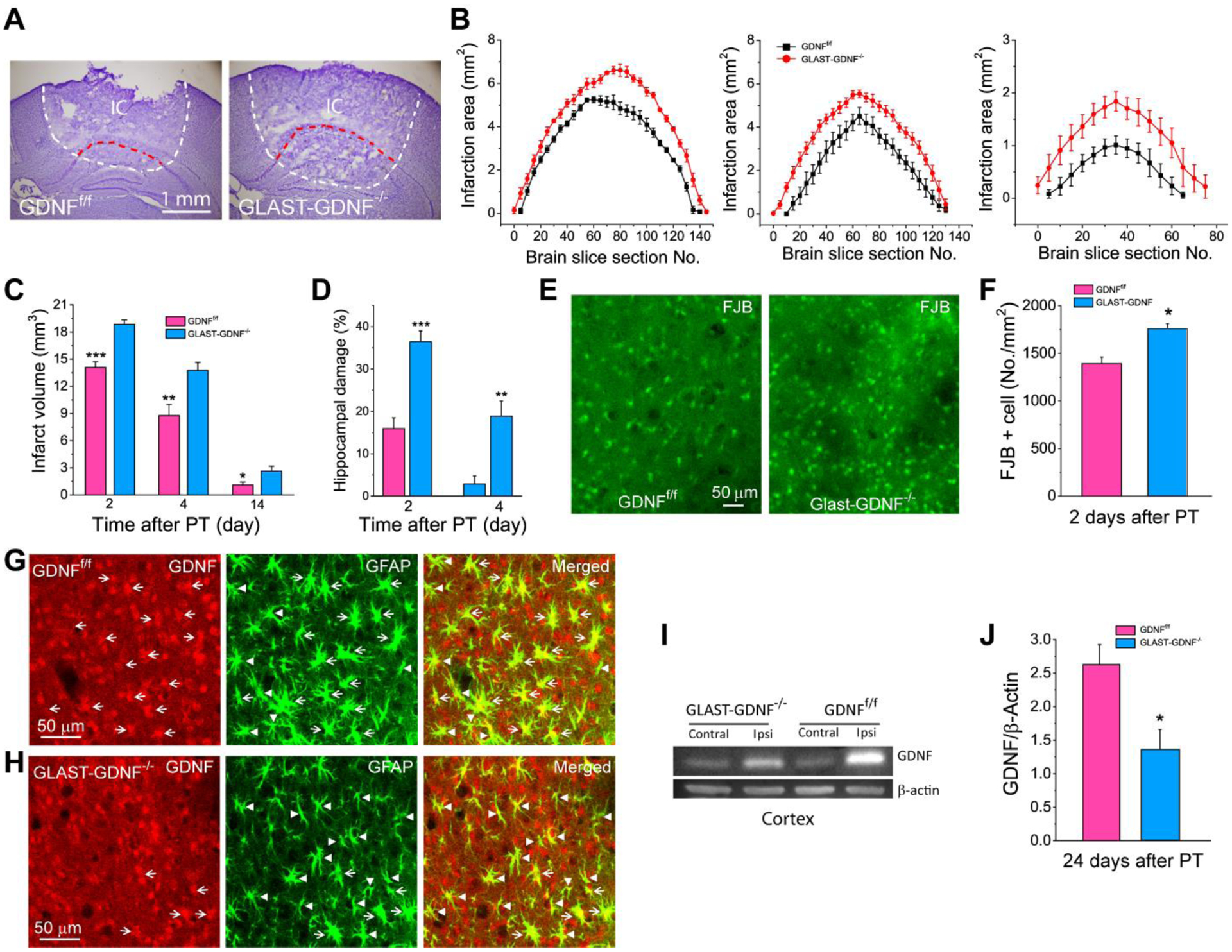
A) Representative Nissl staining of brain sections showing brain infarct areas of GDNFf/f and GLAST-GDNF−/− cKO mice 2 days after PT. The dashed lines outline the infarct regions. IC-ischemic core. B-C) Infarct areas and infarct volumes at different days after PT. Data were averaged from N=6 mice for each group 2 days after PT, N=6 GNDFf/f mice and 7 GLAST-GDNF−/− mice for 4 days after PT, and N=6 mice for each group 14 days after PT. White dash lines outline the total damage area, and the area between red and white dash lines is the area of damaged hipppocampus. D) The volume percentage of hippocampal damage. Data were averaged from N=6 mice for each group 2 days after PT, N=6 GNDFf/f mice and 7 GLAST-GDNF−/− mice for 4 days after PT. E-F) Representative images of FJB staining (D) and the densities of FJB+ cells (E) in the PIR 2 days after PT. N=6 mice for each group and 2 slice for each mouse. G-H) Confocal images of double immunostaining of GFAP and GDNF in control and GDNF cKO mice 4 days after PT. Arrows indicate colocalization of GDNF and GFAP in reactive astrocytes while arrow heads reactive astrocytes without expressing GDNF. I-J) WB analysis of GDNF in the cortex 24 days after PT. N=7 mice for each group. *p<0.05, **p<0.005, ***p<0.0005, t-test.
Deletion of astrocytic GDNF attenuates reactive astrogliosis in the PIR after FIS
Reactive astrogliosis, manifested by hypertrophic morphology with thick process, enhanced proliferative rate and altered gene expression in RAs, is a hallmark of FIS (Choudhury and Ding 2016; Ding, 2014; Burda and Sofroniew, 2014; Zhao and Rempe, 2010). Next, we studied the effect of RAs-derived GDNF on reactive astrogliosis after PT. We conducted immunostaining and confocal imaging to examine Brdu labeled and Ki67 expressing proliferating cells in the PIR (Figure 6A–D). Summary data indicate that GLAST-GDNF−/− cKO mice exhibited a significant reduction in Brdu+ and Ki67+ proliferating cells in the PIR 2 and 4 days after PT (Figure 6E–F, 6G–H). Since GDNF is deleted in RAs, we further studied its effect on cell proliferation and reactive astrogliosis using GFAP and Brdu double immunostaining at different times. Brdu was injected in mice for two times with one day apart before mice were sacrificed 24 h after the second injection to label proliferating cells in this time window (Li et al., 2014). GDNFf/f mice have much more GFAP+ RAs with thick processes and high GFAP immunofluorescent signal than GLAST-GDNF−/− cKO mice, especially 2 and 4 days after PT (Figure 7A–C). Summary data show that GLAST-GDNF−/− cKO mice exhibited a significant decrease in the intensity of GFAP fluorescence signal and the density of GFAP+ RAs as compared with GDNFf/f mice (Figure 7D–E). Moreover, the cKO mice have less Brdu+ cells and GFAP+Brdu+ proliferating RAs (Figure 7F–G), lower ratio of GFAP+ proliferating RAs out of total proliferating cells (i.e., GFAP+Brdu+/Brdu+ ratio) (Figure 7H), and lower ratio of GFAP+ proliferating RAs (i.e., GFAP+Brdu+/GFAP+ ratio) (Figure 7I) than the control mice 2 and 4 days after PT. However, cellular proliferating is largely stabilized at 14 days after PT with no difference between the control and cKO mice (Figure 7E–I) Thus, our results indicate that RAs-derived GDNF can stimulate reactive astrogliosis after FIS by increasing cell proliferation and density of GFAP+ RAs in subacute phase after PT.
Figure 6. Deletion of astrocytic GDNF attenuates cell proliferation in the PIR after PT.
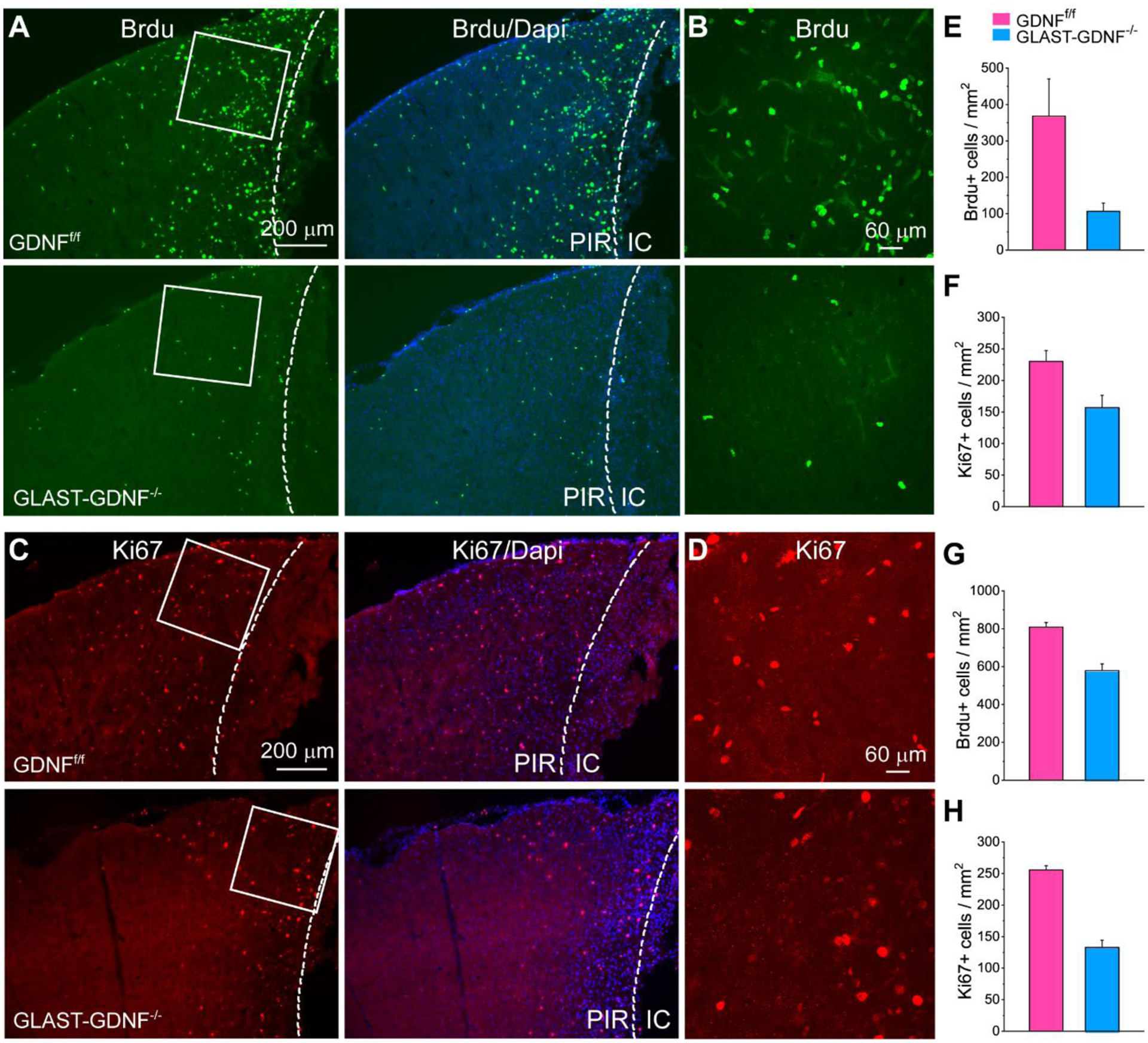
A-D) Fluorescent images of Brdu+ (A-B) and Ki67+ (C-D) cells in the PIR in GDNFf/f and GLAST-GDNF−/− cKO mice 2 days after PT. B and D are the high resolution images of the boxed regions in A and B. PIR-Peri infarct region, IC-ischemic core. E-G) Summary of the densities of Brdu+ and Ki67+ cells in the PIR 2 (E-F) and 4 (G-H) days after PT. Mice were injected with Brdu immediately after PT and sacrificed 2 and 4 days later. N=3 mice and 2 slices for each mouse in each group. *p<0.05, t-test.
Figure 7. Deletion of astrocytic GDNF attenuates reactive gliosis in the PIR after PT.
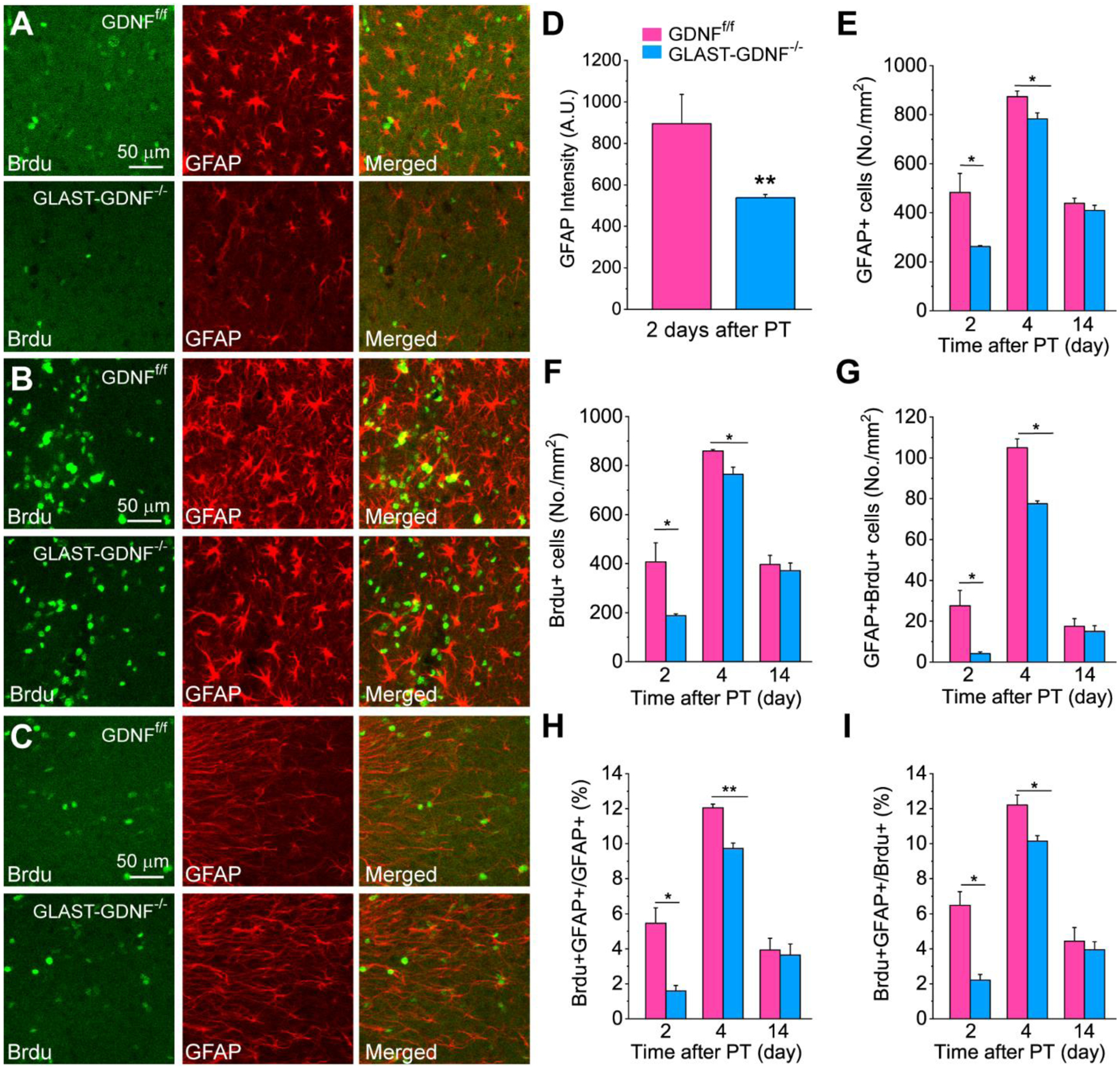
A-C) Double immunostaining images of Brdu and GFAP in the PIR of GDNFf/f and GLAST-GDNF−/− cKO mice at 2 (A), 4 (B) and 14 (C) days after PT. Scale bar is 50 μm in A-C. D) Quantification of GFAP immunofluorescent signal 2 days after PT. E) Densities of GFAP+ RAs. F) Brdu+ cells. G) Brdu+GFAP+ cells. H) The ratio of Brdu+GFAP+ cells to GFAP+ cells. I) the ratio of Brdu+GFAP+ cells to Brdu+ cells. Data in D-I) were averaged values from 3–4 mice and 2 slices for each mouse. *p<0.05, **p<0.005, t-test.
Deletion of astrocytic GDNF reduces G6PD expression in RAs and increases oxidative stress after FIS
Oxidative stress has a large contribution to neuronal death after stroke, and thus affects brain recovery. It is reported that recombinant GDNF can reduce oxidative stress in Parkinson’s disease model (Smith and Cass, 2007). Therefore, we further investigated whether astrocyte-specific deletion of GDNF increased oxidative stress. Reduced glutathione (GSH) is the most abundant antioxidant within all cells and provides the cellular first line of defense against oxidative stress-induced injury (Hwang S et al., 2018; Mejias et al., 2006). The production of GSH is maintained by NADPH which is mainly generated through the pentose phosphate pathway (PPP) and its rate-limiting enzyme G6PD in the oxidative stage. In PPP, G6PD converts G6P to 6-p-gluconolactone and generates NADPH which is required for the regeneration of GSH in antioxidant pathways to reduce oxidative stress (Kuehne et al., 2015; Jeng et al., 2013). Here using double immunostaining of G6PD and GFAP we found that deletion of GDNF in astrocytes largely suppressed the upregulation of G6PD in RAs after PT. G6PD was largely upregulated in RAs in ischemic hemisphere in the GDNFf/f mice (Figure 8A), but G6PD upregulation was suppressed in GLAST-GDNF−/− cKO mice (Figure 8B). Summary data show that the densities of G6PD+ cells (left), G6PD+GFAP+ RAs (middle), and the ratio of G6PD+GFAP+ RAs to total GFAP+ RAs, i.e., G6PD+GFAP+/GFAP+ (right) were much lower in the GLAST-GDNF−/− cKO mice than those in the control mice (Figure 9C). To further determine whether the deletion of GDNF in astrocytes can reduce oxidative stress in vivo, we injected DHE in mice to label reactive oxygen species (ROS). Our results show that the GDNF cKO mice had much higher levels of DHE signal in the PIR than the control mice (Figure 8D–F). Thus, our data demonstrate that RAs-derived GDNF can promote anti-oxidative stress mechanism to exert a neuronal and brain protective effect after FIS.
Figure 8. Deletion of astrocytic GDNF increase oxidative stress.
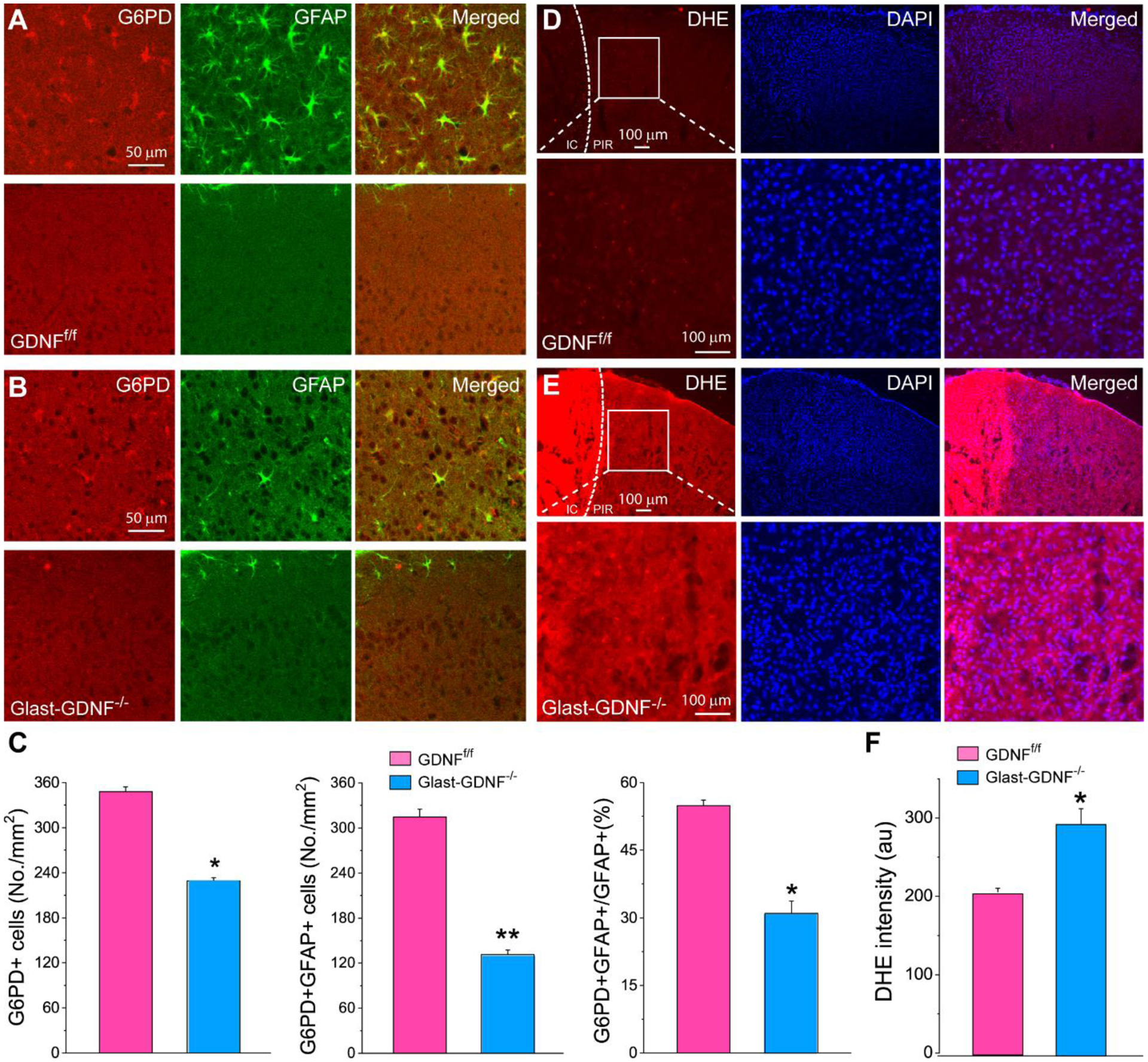
A-B) Double immunostaining of G6PD and GFAP in the PIR (upper panel) and contralateral side (lower panel) of GDNFf/f mice (A) and GLAST-GDNF−/− mice (B) 4 days after PT. Notice that G6PD is highly colocalized with GFAP in PIR in the control mice and the reduction of G6PD expression in the cKO mice. C) Summary data of G6PD+ cells (left), G6PD+GFAP+ RAs (middle) and the ratio of G6PD+RAs to total GFAP+ RAs, i.e., G6PD+GFAP+/GFAP+ (right) in GDNFf/f mice and GLAST-GDNF−/− mice 4 days after PT. The data were averaged from 4 slices of each genotype. D-E) Fluorescent images of DHE and Dapi in the PIR (upper panel) of GDNFf/f mice (D) and GLAST-GDNF−/− mice (E) 2 days after PT. The bottom panels are the high-resolution images of the boxed region of upper panels. Notice the increases in DHE signal in the cKO mice. F) Summary data of DHE fluorescent intensity in the PIR of GDNFf/f mice and GLAST-GDNF−/− mice 2 days after PT. Data were averaged from N=4 mice of each genotype and 4 slices for each mouse. *p<0.05, **p<0.005, t-test.
Figure 9. Deletion of astrocytic GDNF increases behavioral deficits after PT.
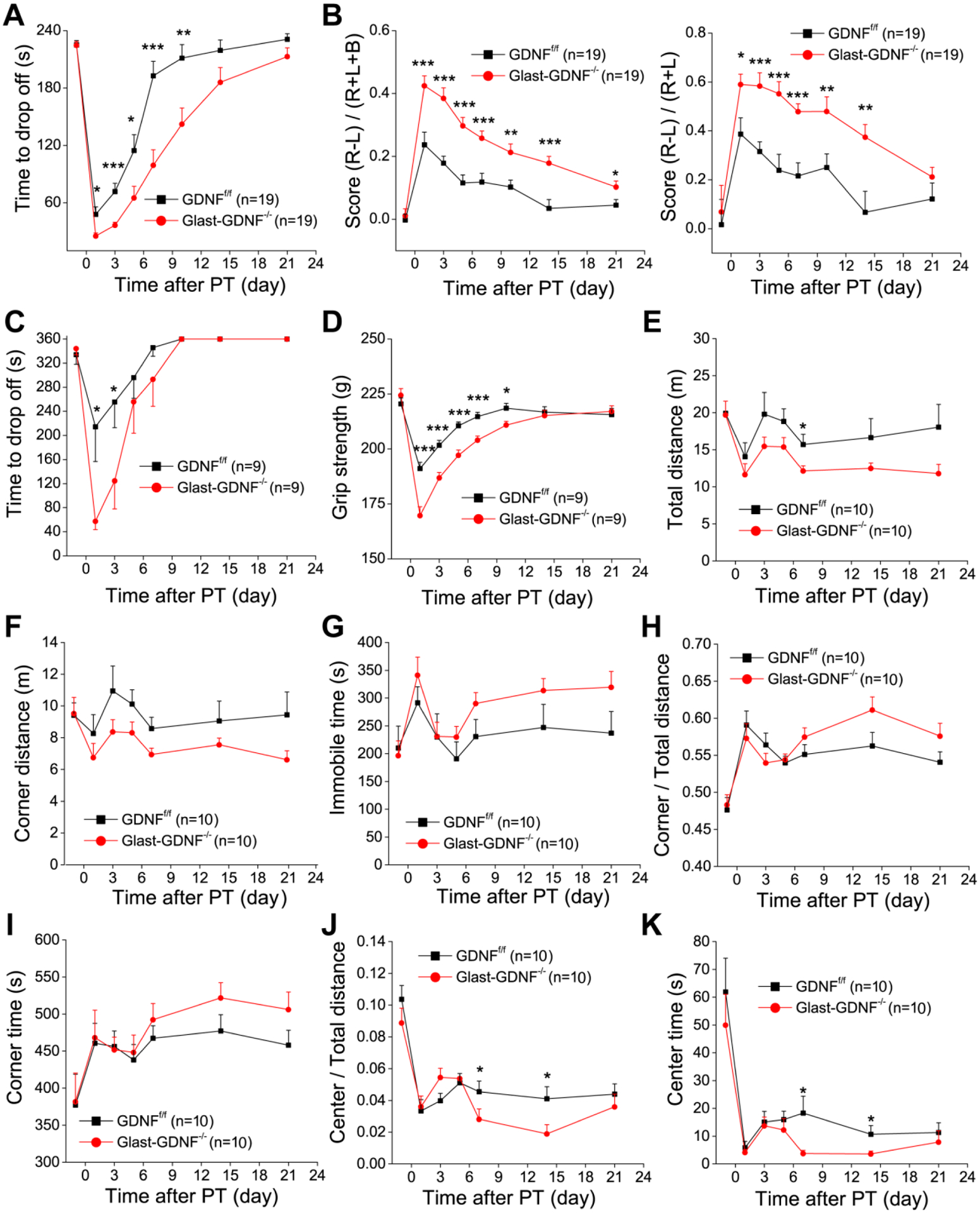
A-D) Four motor behavioral tests, i.e., hanging wire (A), cylinder (B), rotorad (C) and grip force (D) tests were conducted on GDNFf/f and GLAST-GDNF−/− cKO mice before and different times after PT. E-K) Open field test for the evaluation of the general motor activity and anxiety. Time courses of 6 parameters from 10 min open field tests including total travel distance (E), corner distance (F), total immobile time (G), the ratio of corner travel distance to total travel distance (H), the time to explore the corners (I), the ratio of center distance to total distance (J) and the time to explore the center (K). The value of day 0 in each test is the data before PT for ischemic mouse group. Mouse numbers were indicated in the figures. *p<0.05, **p<0.005, ***p<0.0005, ANOVA test.
Deletion of astrocytic GDNF increases motor behavioral deficits after PT
Lastly, we conducted a battery of motor function tests including hanging wire, cylinder, rotorad and grip force tests to determine the effect of GDNF deletion on ischemia-induced behavioral deficits over 3 weeks of the post PT period. We conducted pre-test prior to PT to establish a baseline performance, and conducted the same tests 1, 3, 5, 10, 14 and 21 days after PT. The hanging wire test was performed to evaluate the grasping ability and forelimb strength after ischemia(Li et al., 2004). There was a significant difference between GLAST-GNDF−/− cKO and GDNFf/f control mice at days 1, 3, 5, 7 and 9 after PT (Figure 9A). The cylinder test was conducted to evaluate the asymmetricity of forelimb use for weight shifting during vertical exploration and assess motor deficits after an ischemic stroke (Li, Blizzard, Zeng, DeVries, Hurn, and McCullough, 2004). Data analysis showed that GLAST-GDNF−/− cKO mice exhibited more difference in the frequency of usage between non-impaired and impaired paws than GDNFf/f mice after PT. Statistical differences of performance were observed on days 1, 3, 5, 7, 10 and 14 post-PT (Figure 9B). Rotarod test evaluates motor coordination and balance, and our data show that GLAST-GDNF−/− cKO mice exhibit more deficits at days 1, 3, 5 and 7 post-PT than the control mice (Figure 9C). Grip force test was used to determine four-limbs strengths and our data show that the cKO mice exhibited a weaker grip strength than the control mice (Figure 9D). The open field test evaluates general locomotor activity, anxiety and exploration, but also test cognition-related behavior (Dvorkin, Benjamini, Golanir, 2008). Here we analyzed different parameters for a 10 min exploration period (Figure 9E–L). Our results show that spontaneous motor activity evaluated as total travel distance was not influenced by GDNF deletion in astrocytes before PT, GLAST-GDNF−/− cKO mice exhibited decreased total travel distance and corner travel distance, increased total immobile time, the ratio of corner travel distance to total travel distance and time spent in the corner, but decreased ratio of center travel distance to total travel distance, and center time after PT. These results suggest that deletion of GDNF in astrocytes causes an increase of anxiety-like behavior and a decline of explorative capability. Overall, our study on different behavioral tests indicate that GLAST-GDNF−/− cKO mice have significantly higher motor function deficits and anxiety-like behavior than the GDNFf/f Ctrl mice over the time course of three weeks after PT, suggesting RAs-derived GDNF plays a significant role in promoting brain recovery and improving long-term stroke outcomes.
DISCUSSION
Study on glia-neuron interactions in health and disease faces an inflection point in which cell-specific in vivo experiments are beginning to validate evidence obtained from pharmacological approaches. With the availability of genetic tools such as astrocyte-specific driver transgenic mouse lines and astrocyte-specific adeno-associate virus (AAV) for gene deletion and overexpression (Xie, Wang, Sun, and Ding, 2010; Jahn, Scheller, and Kirchhoff, 2015), dissection of exclusive roles of astrocytes in neuronal circuit and neuronal degeneration/death becomes feasible. GLAST-CreERT2 mediated recombination has been used to express florescent reporters to study the morphology of astrocytes and Ca2+ signaling in astrocytes, and label adult-born granule cell lineage (Bardehle et al., 2013; Paukert et al., 2014; DeCarolis et al., 2013). Increasing evidence indicates a non-cell autonomous effect of astrocytes or RAs on neurodegeneration and death under pathological conditions (Jolly et al., 2011). In current study, we used GLAST-CreERT2 mediated gene deletion to determine whether and how RAs-derived GDNF affects brain damage, neuronal death, reactive astrogliosis, oxidative stress and motor function after PT. Several novel findings were observed using GLAST-GDNF−/− cKO mice and PT-induced FIS mouse model. First, GDNF is highly upregulated in the brain and RAs after PT, and maintains a sustained time during the post-FIS period. Second, GLAST-GDNF−/− cKO mice exhibit reduced adult neurogenesis in hippocampus in normal brain. Third, deletion of GDNF in astrocytes exacerbates brain and hippocampal damage and neuronal death. Fourth, deletion of GDNF in astrocytes reduces reactive astrogliosis after PT. Fifth, deletion of GDNF in astrocyte decreases G6PD and increases ROS production. Sixth, GLAST-GDNF−/− cKO mice exhibit higher motor function deficits than the control mice after PT. These results demonstrated that astrocytes- and/or RAs-derived GDNF is beneficial in protecting against neuronal death and brain damage after FIS, and improving long-term stroke outcomes. These findings indicate the involvement of endogenous GDNF in astrocytes/RAs as a growth factor in these processes.
Although the effects of recombinant GDNF on brain protection in stroke were investigated (Abe and Hayashi, 1997; Kitagawa et al., 1999; Wei, Wu, and Cao, 2000; Miyazaki, Nagashima, Okuma, and Nomura, 2001; Arvidsson et al., 2001; Kuric, Wieloch, and Ruscher, 2013), compared with the current study, there are a few weaknesses in these studies. First, a local delivery of recombinant GDNF protein only affects a small region of tissue surrounding the injection site. Second, most studies evaluated the effects on brain infarction and cell death in the acute phase, but the effects on long-term neural regeneration and motor functional recovery have not been studied. Third, a single time administration of recombinant GDNF cannot maintain its effect for a prolonged time, thus chronic infusion is needed. Fourth, GDNF administration is not cell-type specific, therefore, leaving the respective roles of neuron- or astrocyte-derived GDNF in brain protection and recovery unclear. Fifth, significant toxicity effect was observed after continuous administration of recombinant GDNF in primate PD model (Luz, Mohr, and Fibiger, 2016). Genetic strategies such as viral transduction to increase local production of GDNF were also tested for stroke therapy (Yagi et al., 2000; Harvey et al., 2003; Tsai et al., 2000; Arvidsson et al., 2003; Hermann et al., 2001); however, astrocyte-specific viral overexpression of GDNF has not been reported in stroke.
In normal adult brain, GDNF is expressed at very low levels; it is mostly expressed in neurons and absent from or expressed little in astrocytes (Miyazaki, Nagashima, Okuma, and Nomura, 2001; Hidalgo-Figueroa et al., 2012; Kuric, Wieloch, and Ruscher, 2013). GDNF can be secreted to extracellular space as glycosylated mature protein to mediate cellular responses through GDNF family ligand receptors (GFRs) (Luz, Mohr, and Fibiger, 2016). Cultured cortical astrocytes and neurons both express GFRs (Nicole et al., 2001). In current study, we observed GDNF is dramatically increased for a prolonged time after PT, suggesting GDNF is a stress-responsive gene, and the secreted GDNF can act on neurons to mediate endogenous protective effect after FIS. Moreover, GNDF is also largely increased in RAs. Glial scar formation due to reactive astrogliosis is the hallmark of FIS. Following FIS, astrocytes become activated and these RAs exhibit spatiotemporal dependent changes in proliferation, morphology, and gene expression, which highly correlates with the progression of brain and neuronal remodeling and behavioral recovery (Ding, 2014; Choudhury and Ding 2016; Burda and Sofroniew, 2014; Anderson, Ao and Sofroniew, 2014; Li et al., 2014). In the chronic phase, the morphology of RAs remains stable, the proliferation of RAs ceases and glial scar is stabilized, causing substantial tissue remodeling and permanent structural changes of the PIR (Barreto, Sun, Xu, and Giffard, 2011; Li et al., 2014; Ding, 2014; Choudhury and Ding 2016; Burda and Sofroniew, 2014; Voskuhl et al., 2009). Our results that GLAST-CreERT2 mediated GDNF deletion caused more neuronal death, larger brain infarction, more ROS and more severe motor behavioral deficits indicate that endogenous extracellular GDNF derived from RAs plays a role in brain recovery and improvement in long-term outcomes after FIS, highlighting the non-cell autonomous effect of RAs on neuroprotection and motor function recovery through GDNF release.
Neurogenesis in the adult brain are from two neurogenic regions in the brain, the SGZ of DG in the hippocampus and the subventricular zone (SVZ) along the walls of the lateral ventricle (Lois and Alvarez-Buylla, 1993; Gage et al., 1998). The unique microenvironments (i.e., ‘niches’) in the two regions including soluble factors, membrane-bound molecules and the extracellular matrix permit stem cell self-renewal and progenitor differentiation into neurons in adult stage. Neurogenesis and the survival of newly generated neurons can be regulated by various factors. GLAST is an astrocyte-specific glutamate transporter and is also expressed in type I neural stem cell. GLAST-lineage radial glia-like cells (RGCs) contribute to adult hippocampal neurogenesis (DeCarolis et al., 2013). In current study, we observed that GDNF is reduced in hippocampus in the GLAST-GDNF−/− cKO mice under normal conditions albeit without statistical significance. We also found that the cKO mice exhibit reduced adult neurogenesis in DG under normal conditions, suggesting GDNF in GLAST-lineage RGCs is an important growth factor to facilitate adult neurogenesis. The results of neurogenesis and GDNF levels are not contradictive, since in normal adult brain, GDNF is expressed at very low levels and it is mostly expressed in neurons and little in astrocytes (Miyazaki, Nagashima, Okuma, and Nomura, 2001; Hidalgo-Figueroa et al., 2012; Kuric, Wieloch, and Ruscher, 2013). On the other hands, growing evidence suggests that FIS increases neurogenesis in the SVZ and SGZ in DG, where RAs play an important role (Tobin et al., 2014; Young et al., 2013). An ischemic stroke causes substantial reactive astrogliosis in SVZ and there is an association between endogenous neurogenesis and improved stroke outcomes (Lagace, 2012), therefore, RAs could play a role in stroke recovery through modulating neurogenesis. Stroke also elicits a latent neurogenic program in striatal astrocytes in a mouse model (Magnusson et al., 2014). Striatal astrocytes produce neuroblasts and Notch 1 signaling is reduced in astrocytes after a stroke, and furthermore, blocking Notch signaling triggers astrocytes in the striatum and the medial cortex to enter a neurogenic program even in the absence of a stroke, indicating that attenuated Notch 1 signaling is necessary for neurogenesis by striatal astrocytes. In current study, we found that GLAST-GDNF−/− cKO mice exhibit reduced adult neurogenesis in DG under normal conditions and increased motor function deficits after PT, suggesting GLAST-CreERT2-mediated GDNF deletion could cause the reduction of stroke-induced neurogenesis, and subsequently worsen long-term brain recovery. However, although GDNF is increased after stroke in our model, giving the damage of hippocampus (especially in the cKO mice), it is not feasible to accurately evaluate hippocampal neurogenesis after stroke.
In addition to having proliferative capacity, RAs have many other stem cell-like properties (Buffo et al., 2008; Sirko et al., 2013; Robel, Berninger, and Gotz, 2011; Dimou, 2014; Shimada, LeComte, Granger, Quinlan, and Spees, 2012; LeComte, Shimada, Sherwin, and Spees, 2015; Shimada, Borders, Aronshtam, and Spees, 2011; Gotz, Sirko, Beckers, and Irmler, 2015). They express neural stem cell markers such as nestin, Sox2 and DCX (Ohab, Fleming, Blesch, and Carmichael, 2006; Magnusson et al., 2014; Shimada, Borders, Aronshtam, and Spees, 2011; Shimada et al., 2012). RAs can also potentially transform into neurons under specific conditions such as an overexpression of neurogenic factors. In this regard, RAs can be converted to neuroblasts and neurons by forced expression of a single transcriptional factor such as Sox2 (Su et al., 2014), neurogenin-2 (Berninger et al., 2007; Heinrich et al., 2010), NeuroD1 (Guo, Zhang, Wu, Chen, Wang, and Chen, 2014), or a combination of multiple transcriptional factors such as ASCL1, LMX1B and NURR1 (Addis et al., 2011). In our study, we found that GLAST-GDNF−/− cKO mice exhibit a reduced proliferative rate of RAs, decreased GFAP expression levels and enhanced motor function deficits compared with the control mice, indicating that the RAs-derived GDNF affects dynamics of reactive astrogliosis and stem cell properties through a cell autonomous effect. The results corroborate the data of reduced adult neurogenesis in the GLAST-GDNF−/− cKO mice under normal conditions, and also suggest that reduced reactive astrogliosis is associated with worsening motor function recovery in the cKO mice.
GDNF may facilitate neuronal survival in multiple ways. Data from recombinant GDNF suggest that GDNF could protect neurons through reducing oxidative stress via transcriptional regulation of the glutathione synthesis (Iwata-Ichikawa et al., 1999) and apoptosis via caspase-3 dependent pathway (Kilic et al., 2003), upregulating anti-apoptotic Bcl-2 and Bcl-Xl levels (Kitagawa et al., 1998; Sawada et al., 2000), and potentiating and prolonging the activation of p-Akt pathway (Jin et al., 2003). In current study, in parallel with reduced reactive astrogliosis, we found that deletion of GDNF in astrocytes significantly reduced G6PD in RAs and increased ROS. G6PD is the key cytoprotective enzyme in PPP and is of central importance to provide NADPH, the major source of reducing equivalent of a cell. Activation of PPP has been considered as first line of defensive response against oxidative stress-induced injury, where G6PD plays a critical role (Hwang S et al., 2018; Mejias et al., 2006). The generation of antioxidant GSH requires NADPH (Kuehne et al., 2015; Jeng et al., 2013), thus high G6PD expression levels in RAs indicate high NADPH levels and therefore high anti-oxidative effect. Our study provide strong evidence that RAs derived GDNF can protect neurons and brain through activating an antioxidant mechanism via PPP.
Although GLAST-CreERT2 driver line is widely used to mediate gene deletion or overexpression in astrocytes, a drawback of this driver line is the relative low efficiency of gene recombination. Other CreERT2 driver lines for genetic targeting of astrocytes are also available. In this regard, GFAP-CreERT2 and ALDH1L1-CreERT2 lines were developed for studying astrocyte-specific gene expression and deletion (Ganat et al., 2006; Winchenbach et al., 2016). These driver lines exhibit brain region-dependent recombination and have advantages and disadvantages. For example, recombination in GLAST-CreERT2 mice dominates forebrain regions, while the GFAP-CreERT2 mice exhibit high recombination efficiency in the hindbrain (Jahn, Scheller, and Kirchhoff, 2015). GFAP-CreERT2 and GLAST-CreERT2 mice suffer from recombination in radial glia of the neurogenic niches (Mori et al., 2006; DeCarolis et al., 2013; Hirrlinger et al., 2006). The recently developed ALDH1L1-CreERT2 lines have high recombination efficiency (Srinivasan et al., 2016; Winchenbach et al., 2016). ALDH1L1 catalyzes the conversion of 10-formyltetrahydrofolate, nicotinamide adenine dinucleotide phosphate (NADP+) and water to tetrahydrofolate, NADPH and CO2. ALDH1L1 expression is tissue-specific and is highly and specifically expressed in astrocytes in CNS (Cahoy et al., 2008). Nevertheless, ALDH1L1 is also expressed in neural stem cells in the brain (Foo and Dougherty, 2013). Thus, GLAST, GFAP and ALDH1L1 promoters are all active in neural stem cells. On the other hand, ALDH1L1 is also highly expressed in the liver (Cahoy et al., 2008; Cook, Lloyd, and Wagner, 1991), thus ALDH1L1-CreERT2 line is probably more suitable for biosensor expression rather than for gene deletion in astrocytes.
Brain have a remarkable capacity for spontaneous recovery after FIS, but its mechanism is largely unknown. Understanding its mechanism will help us to identify potential strategies to facilitate brain recovery and improve stroke outcomes. In current study, we used the loss-of-function gene deletion approach to study the role and mechanism of astrocytic GDNF in neuronal and brain protection. In future, it is important to use gain-of-function tools such as astrocyte-specific overexpression of GDNF by AAV transduction to study the effect of RAs-derived GDNF on neuronal protection and functional recovery. It is also important to determine whether astrocytic deletion or overexpression of GDNF affects neuronal function such as synaptic transmission and action potential firing under normal conditions. This will further help to understand non-cell autonomous effect of astrocyte on neuronal function under normal condition as well as on neuronal degeneration under pathological conditions. It will be also important to investigate how GDNF is released from RAs. Positive results will further demonstrate that targeting RAs is a potential strategy in stroke restorative therapies.
Supplementary Material
Figure S1. GLAST-CreERT2-mediated gene recombination. A-D) Fluorescent images of s100b and tdTomato in the cortex (A-B) and hippocampus (C-D). The bottom panels are the high resolution images of the upper panels. Four-week old GLAST-CreERT2:GCaMP5-IRES-tdTomatof/f mice were injected with TAM (100 mg/Kg) for 5 consecutive days and sacrificed 2 weeks later.
Figure S2. Increased Cre activity in GLAST-CreERT2 mice after FIS. Fluorescent images of immunostaining of Cre and GFAP in the non-ischemic (A-B) and ischemic (C-D) sides of brain. Cre expression in the cortex of ischemic side were largely increased in GLAST-CreERT2 mice two days after PT. Notice many GFAP+ RAs are colocalized with Cre in the penumbra in ischemic side, and there are some Cre+ cells in non-ischemic side, but the GFAP expression is low.
Main points.
Astrocytic GDNF facilitates adult neurogenesis.
Astrocytic GDNF attenuates brain damage, neuronal death and oxidative stress after focal ischemic stroke (FIS).
Astrocytic GDNF promotes reactive astrogliosis and motor functional recovery after FIS.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Health [National Institute of Neurological Disorders and Stroke (NINDS) grants R01NS069726 and R01NS094539 to SD] and the America Heart Association [Midwest Affiliate Grant in Aid award (16GRANT31280014) to SD]. We acknowledge Ms. Samantha Ding for her help with making TOCI.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Abe K, Hayashi T. 1997. Expression of the glial cell line-derived neurotrophic factor gene in rat brain after transient MCA occlusion. Brain Research 776:230–234. [DOI] [PubMed] [Google Scholar]
- Addis RC, Hsu FC, Wright RL, Dichter MA, Coulter DA, Gearhart JD. 2011. Efficient Conversion of Astrocytes to Functional Midbrain Dopaminergic Neurons Using a Single Polycistronic Vector. PLoS ONE 6:e28719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. 2002. The GDNF family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383–394. [DOI] [PubMed] [Google Scholar]
- Anderson MA, Ao Y, Sofroniew MV. 2014. Heterogeneity of reactive astrocytes. Neuroscience Letters 565:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Kokaia Z, Airaksinen MS, Saarma M, Lindvall O. 2001. Stroke induces widespread changes of gene expression for glial cell line-derived neurotrophic factor family receptors in the adult rat brain. Neuroscience 106:27–41. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Kirik D, Lundberg C, Mandel RJ, Andsberg G, Kokaia Z, Lindvall O. 2003. Elevated GDNF levels following viral vector-mediated gene transfer can increase neuronal death after stroke in rats. Neurobiology of Disease 14:542–556. [DOI] [PubMed] [Google Scholar]
- Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Gotz M. 2013. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nature Neuroscience 16:580. [DOI] [PubMed] [Google Scholar]
- Barreto GE, Sun X, Xu L, Giffard RG. 2011. Astrocyte Proliferation Following Stroke in the Mouse Depends on Distance from the Infarct. PLoS ONE 6:e27881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B, Gotz M. 2007. Functional Properties of Neurons Derived from In Vitro Reprogrammed Postnatal Astroglia. The Journal of Neuroscience 27:8654–8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH. 2009. In Vivo Voltage-Sensitive Dye Imaging in Adult Mice Reveals That Somatosensory Maps Lost to Stroke Are Replaced over Weeks by New Structural and Functional Circuits with Prolonged Modes of Activation within Both the Peri-Infarct Zone and Distant Sites. The Journal of Neuroscience 29:1719–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. 2008. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. PNAS 105:3581–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda J, Sofroniew M. 2014. Reactive Gliosis and the Multicellular Response to CNS Damage and Disease. Neuron 81:229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. 2008. A Transcriptome Database for Astrocytes, Neurons, and Oligodendrocytes: A New Resource for Understanding Brain Development and Function. The Journal of Neuroscience 28:264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Oyarzabal EA, Sung YF, Chu CH, Wang Q, Chen SL, Lu RB, Hong JS. 2015. Microglial regulation of immunological and neuroprotective functions of astroglia. GLIA 63:118–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ai Y, Slevin JR, Maley BE, Gash DM. 2005. Progenitor proliferation in the adult hippocampus and substantia nigra induced by glial cell line-derived neurotrophic factor. Experimental Neurology 196:87–95. [DOI] [PubMed] [Google Scholar]
- Chouchane M, Costa MR. 2012. Cell therapy for stroke: use of local astrocytes. Frontiers in Cellular Neuroscience 6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury GR, Ding S. 2016. Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiology of Disease 85:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Lopez-Valdes HE, Overman JJ, Charles AC, Brennan KC, Thomas Carmichael S. 2013. Multimodal examination of structural and functional remapping in the mouse photothrombotic stroke model. J Cereb Blood Flow Metab 33:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RJ, Lloyd RS, Wagner C. 1991. Isolation and characterization of cDNA clones for rat liver 10-formyltetrahydrofolate dehydrogenase. J Biol Chem 266:4965–4973. [PubMed] [Google Scholar]
- DeCarolis NA, Mechanic M, Petrik D, Carlton A, Ables JL, Malhotra S, Bachoo R, Gotz M, Lagace DC, Eisch AJ. 2013. In vivo contribution of nestin- and GLAST-lineage cells to adult hippocampal neurogenesis. Hippocampus 23:708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou LGt. 2014. Glial Cells as Progenitors and Stem Cells: New Roles in the Healthy and Diseased Brain. Physiol Rev 94:709–737. [DOI] [PubMed] [Google Scholar]
- Ding S, Wang T, Cui W, Haydon PG, Ding S, Wang T, Cui W, Haydon PG. 2009. Photothrombosis ischemia stimulates a sustained astrocytic Ca2+ signaling in vivo. GLIA 57:767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S 2014. Dynamic reactive astrocytes after focal ischemia. Neural Regenerative Research 9:2048–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorkin A, Benjamini Y, Golani I. 2008. Mouse Cognition-Related Behavior in the Open-Field: Emergence of Places of Attraction. PloS Computational Biology 4: e1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Bonvento G. 2008. Targeted Activation of Astrocytes: A Potential Neuroprotective Strategy. Mol Neurobiol 38:231–241. [DOI] [PubMed] [Google Scholar]
- Foo LC, Dougherty JD. 2013. Aldh1L1 is expressed by postnatal neural stem cells in vivo. GLIA 61:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo L, Allen N, Bushong E, Ventura P, Chung WS, Zhou L, Cahoy J, Daneman R, Zong H, Ellisman M, Barres B. 2011. Development of a Method for the Purification and Culture of Rodent Astrocytes. Neuron 71:799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Sirko S, Beckers J, Irmler M. 2015. Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, In vitro potential, and Genome-wide expression analysis. GLIA 63:1452–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. 1998. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol 36:249–266. [DOI] [PubMed] [Google Scholar]
- Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, Ment LR, Vaccarino FM. 2006. Early Postnatal Astroglial Cells Produce Multilineage Precursors and Neural Stem Cells In Vivo. The Journal of Neuroscience 26:8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee J, Smith N, Fernandez F, Economo M, Brunert D, Rothermel M, Morris S, Talbot A, Palumbos S, Ichida J, Shepherd J, West P, Wachowiak M, Capecchi M, Wilcox K, White J, Tvrdik P. 2014. Imaging Activity in Neurons and Glia with a Polr2a-Based and Cre-Dependent GCaMP5G-IRES-tdTomato Reporter Mouse. Neuron 83:1058–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichman AJ, Carmichael ST. 2014. Astrocytic therapies for neuronal repair in stroke. Neuroscience Letters 565:47–52. [DOI] [PubMed] [Google Scholar]
- Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. 2014. In-Vivo Direct Reprogramming of Reactive Glial Cells into Functional Neurons after Brain Injury and in an Alzheimer’s Disease Model. Cell Stem Cell 14:188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. 2007. The tripartite synapse: roles for gliotransmission in health and disease. Trends in Molecular Medicine 13, 54–63. [DOI] [PubMed] [Google Scholar]
- Harvey BK, Chang CF, Chiang YH, Bowers WJ, Morales M, Hoffer BJ, Wang Y, Federoff HJ. 2003. HSV amplicon delivery of glial cell line-derived neurotrophic factor is neuroprotective against ischemic injury. Experimental Neurology 183:47–55. [DOI] [PubMed] [Google Scholar]
- Haydon PG. 2001. GLIA: listening and talking to the synapse. Nature Reviews Neuroscience 2:185–193. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. 2006. Astrocyte Control of Synaptic Transmission and Neurovascular Coupling. Physiol Rev 86:1009–1031. [DOI] [PubMed] [Google Scholar]
- Heinrich C, Blum R, Gascon S, Masserdotti G, Tripathi P, Saínchez R, Tiedt S, Schroeder T, Gotz M, Berninger B. 2010. Directing Astroglia from the Cerebral Cortex into Subtype Specific Functional Neurons. PLoS Biol 8:e1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann DM, Kilic E, Kugler S, Isenmann S, Bañhr M. 2001. Adenovirus-Mediated GDNF and CNTF Pretreatment Protects against Striatal Injury Following Transient Middle Cerebral Artery Occlusion in Mice. Neurobiology of Disease 8:655–666. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Figueroa Ma, Bonilla S, Gutierrez F, Pascual A, Lopez-Barneo J. 2012. GDNF Is Predominantly Expressed in the PV+ Neostriatal Interneuronal Ensemble in Normal Mouse and after Injury of the Nigrostriatal Pathway. The Journal of Neuroscience 32:864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirrlinger PG, Scheller A, Braun C, Hirrlinger J, Kirchhoff F. 2006. Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen-inducible DNA recombinase variant CreERT2. GLIA 54:11–20. [DOI] [PubMed] [Google Scholar]
- Hodge R, Kahoud R, Hevner R. 2012. Transcriptional control of glutamatergic differentiation during adult neurogenesis. Cell Mol Life Sci 69:2125–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. 2006. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res 84:1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Mruk K, Rahighi S, Raub AG, Chen CH, Dorn LE, Horikoshi N, Wakatsuki S, Chen JK, Mochly-Rosen D. 2018. Correcting glucose-6-phosphate dehydrogenase deficiency with a small-molecule activator. Nat Commun. 9:4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata-Ichikawa E, Kondo Y, Miyazaki I, Asanuma M, Ogawa N. 1999. Glial Cells Protect Neurons Against Oxidative Stress via Transcriptional Up-Regulation of the Glutathione Synthesis. Journal of Neurochemistry 72:2334–2344. [DOI] [PubMed] [Google Scholar]
- Jahn HM, Scheller A, Kirchhoff F. 2015. Genetic control of astrocyte function in neural circuits. Frontiers in Cellular Neuroscience 9:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng W, Loniewska MM, Wells PG. 2013. Brain glucose-6-phosphate dehydrogenase protects against endogenous oxidative DNA damage and neurodegeneration in aged mice. ACS Chem Neurosci. 4:1123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CJ, Demol F, Bauwens R, Kooijman R, Massie A, Villers As, Ris L, De Keyser J. 2016. Astrocytic Adrenergic Receptor Gene Deletion Affects Memory in Aged Mice. PLoS ONE 11:e0164721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G, Omori N, Li F, Nagano I, Manabe Y, Shoji M, Abe K. 2003. Protection against ischemic brain damage by GDNF affecting cell survival and death signals. International Congress Series 1252:221–231. [DOI] [PubMed] [Google Scholar]
- Jolly S, Journiac N, Naudet Fdr, Gautheron V, Mariani J, Vernet-der Garabedian B. 2011. Cell-Autonomous and Non-Cell-Autonomous Neuroprotective Functions of RORα in Neurons and Astrocytes during Hypoxia. The Journal of Neuroscience 31:14314–14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Dietz Gunnar PH, Bañhr M. 2003. Intravenous TAT-GDNF Is Protective After Focal Cerebral Ischemia in Mice. Stroke 34:1304–1310. [DOI] [PubMed] [Google Scholar]
- Kimelberg H, Nedergaard M. 2010. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics 7:338–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Bjorklund A. 2004. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci 7:105–110. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Hayashi T, Mitsumoto Y, Koga N, Itoyama Y, Abe K. 1998. Reduction of Ischemic Brain Injury by Topical Application of Glial Cell Line Derived Neurotrophic Factor After Permanent Middle Cerebral Artery Occlusion in Rats. Stroke 29:1417–1422. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Sasaki C, Zhang WR, Sakai K, Shiro Y, Warita H, Mitsumoto Y, Mori T, Abe K. 1999. Induction of glial cell line-derived neurotrophic factor receptor proteins in cerebral cortex and striatum after permanent middle cerebral artery occlusion in rats. Brain Research 834:190–195. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ahlenius H, Thored P, Kobayashi R, Kokaia Z, Lindvall O. 2006. Intracerebral Infusion of Glial Cell Line-Derived Neurotrophic Factor Promotes Striatal Neurogenesis After Stroke in Adult Rats. Stroke 37:2361–2367. [DOI] [PubMed] [Google Scholar]
- Kuehne A , Emmert H , Soehle J, Winnefeld M , Fischer F, Wenck H , Gallinat S, Terstegen L, Lucius R, Hildebrand J , Zamboni N. 2015. Acute activation of oxidative pentose phosphate pathway as first-line response to oxidative stress in human skin Cells. Mol Cell. 59: 359–71. [DOI] [PubMed] [Google Scholar]
- Kuric E, Wieloch T, Ruscher K. 2013. Dopamine receptor activation increases glial cell line-derived neurotrophic factor in experimental stroke. Experimental Neurology 247:202–208. [DOI] [PubMed] [Google Scholar]
- Lagace DC. 2012. Does the endogenous neurogenic response alter behavioral recovery following stroke? Behavioural Brain Research 227:426–432. [DOI] [PubMed] [Google Scholar]
- LeComte MD, Shimada IS, Sherwin C, Spees JL. 2015. Notch1-STAT3-ETBR signaling axis controls reactive astrocyte proliferation after brain injury. PNAS 112:8726–8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledda F, Paratcha G, Sandoval-Guzman T, Ibanez CF. 2007. GDNF and GFRα1 promote formation of neuronal synapses by ligand-induced cell adhesion. Nat Neurosci 10:293–300. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang N, Lin H, Yu Y, Cai QM, Li H, Zhang N, Lin H, Yu Y, Cai QM. 2014. Histological, cellular and behavioral assessments of stroke outcomes after photothrombosis-induced ischemia in adult mice. BMC Neuroscience 15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xie Y, Zhang N, Yu Y, Zhang Q, Ding S. 2015. Disruption of IP3R2-mediated Ca2+ signaling pathway in astrocytes ameliorates neuronal death and brain damage while reducing behavioral deficits after focal ischemic stroke. Cell Calcium 58:565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang N, Sun G, Ding S. 2013. Inhibition of the group I mGluRs reduces acute brain damage and improves long-term histological outcomes after photothrombosis-induced ischaemia. ASN NEURO 5:95–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. 2004. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Experimental Neurology 187:94–104. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. 1993. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130–1132. [DOI] [PubMed] [Google Scholar]
- Lobsiger CS, Cleveland DW. 2007. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci 10:1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. 1993. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. PNAS 90:2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz M, Mohr E, Fibiger HC. 2016. GDNF-induced cerebellar toxicity: A brief review. NeuroToxicology 52:46–56. [DOI] [PubMed] [Google Scholar]
- Magnusson JP, Goritz C, Tatarishvili J, Dias DO, Smith EMK, Lindvall O, Kokaia Z, Frisen J. 2014. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science 346:237–241. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. 2006. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neuro 2:679–689. [DOI] [PubMed] [Google Scholar]
- Marchetto MCN, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. 2008. Non-Cell-Autonomous Effect of Human SOD1G37R Astrocytes on Motor Neurons Derived from Human Embryonic Stem Cells . Cell Stem Cell 3:649–657. [DOI] [PubMed] [Google Scholar]
- Mejías R, Villadiego J, Pintado CO, Vime PJ, Gao L, Toledo-Aral JJ, Echevarría M, López-Barneo J. 2006. Neuroprotection by transgenic expression of glucose-6-phosphate dehydrogenase in dopaminergic nigrostriatal neurons of mice. J Neurosci. 26:4500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki H, Nagashima K, Okuma Y, Nomura Y. 2001. Expression of glial cell line-derived neurotrophic factor induced by transient forebrain ischemia in rats. Brain Research 922:165–172. [DOI] [PubMed] [Google Scholar]
- Mori T, Tanaka K, Buffo A, Wurst W, Kuhn R, Gotz M. 2006. Inducible gene deletion in astroglia and radial gliaΓÇöA valuable tool for functional and lineage analysis. GLIA 54:21–34. [DOI] [PubMed] [Google Scholar]
- Mostany R, Chowdhury TG, Johnston DG, Portonovo SA, Carmichael ST, Portera-Cailliau C. 2010. Local Hemodynamics Dictate Long-Term Dendritic Plasticity in Peri-Infarct Cortex. The Journal of Neuroscience 30:14116–14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Corbett D. 2009. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci 10:861–872. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Hida H, Shimano Y, Fujimoto I, Hashitani T, Kumazaki M, Sakurai T, Nishino H. 2001. GDNF is a major component of trophic activity in DA-depleted striatum for survival and neurite extension of DAergic neurons. Brain Research 916:76–84. [DOI] [PubMed] [Google Scholar]
- Nicole O, Ali C, Docagne F, Plawinski L, MacKenzie ET, Vivien D, Buisson A. 2001. Neuroprotection Mediated by Glial Cell Line-Derived Neurotrophic Factor: Involvement of a Reduction of NMDA-Induced Calcium Influx by the Mitogen-Activated Protein Kinase Pathway. The Journal of Neuroscience 21:3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. 2006. A Neurovascular Niche for Neurogenesis after Stroke. The Journal of Neuroscience 26:13007–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. 2008. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci 11:755–761. [DOI] [PubMed] [Google Scholar]
- Paukert M, Agarwal A, Cha J, Doze V, Kang J, Bergles D. 2014. Norepinephrine Controls Astroglial Responsiveness to Local Circuit Activity. Neuron 82:1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Rieker C, Fuchs S, Bleckmann D, Esposito MS, Botta P, Goldstein C, Bernhard M, Galimberti I, Muller M, Luthi A, Arber S, Bouwmeester T, van der Putten H, Di Giorgio FP. 2014. Transneuronal propagation of mutant huntingtin contributes to non-cell autonomous pathology in neurons. Nat Neurosci 17:1064–1072. [DOI] [PubMed] [Google Scholar]
- Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. 2007. Variations in Promoter Activity Reveal a Differential Expression and Physiology of Glutamate Transporters by Glia in the Developing and Mature CNS. The Journal of Neuroscience 27:6607–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel S, Berninger B, Gotz M. 2011. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci 12:88–104. [DOI] [PubMed] [Google Scholar]
- Rocha SM, Cristovaúo AC, Campos FL, Fonseca CP, Baltazar G. 2012. Astrocyte-derived GDNF is a potent inhibitor of microglial activation. Neurobiology of Disease 47:407–415. [DOI] [PubMed] [Google Scholar]
- Sawada H, Ibi M, Kihara T, Urushitani M, Nakanishi M, Akaike A, Shimohama S. 2000. Neuroprotective Mechanism of Glial Cell Line-Derived Neurotrophic Factor in Mesencephalic Neurons. Journal of Neurochemistry 74:1175–1184. [DOI] [PubMed] [Google Scholar]
- Shang J, Deguchi K, Yamashita T, Ohta Y, Zhang H, Morimoto N, Liu N, Zhang X, Tian F, Matsuura T, Funakoshi H, Nakamura T, Abe K. 2010. Antiapoptotic and antiautophagic effects of glial cell line-derived neurotrophic factor and hepatocyte growth factor after transient middle cerebral artery occlusion in rats. J Neurosci Res 88:2197–2206. [DOI] [PubMed] [Google Scholar]
- Shimada IS, Borders A, Aronshtam A, Spees JL. 2011. Proliferating Reactive Astrocytes Are Regulated by Notch-1 in the Peri-Infarct Area After Stroke. Stroke 42:3231–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada IS, LeComte MD, Granger JC, Quinlan NJ, Spees JL. 2012. Self-Renewal and Differentiation of Reactive Astrocyte-Derived Neural Stem/Progenitor Cells Isolated from the Cortical Peri-Infarct Area after Stroke. The Journal of Neuroscience 32:7926–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneider NA, Brown MN, Smith C, Pickel J, Alvarez FJ. 2009. Gamma motor neurons express distinct genetic markers at birth and require muscle spindle-derived GDNF for postnatal survival. Neural Development 4:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirko S, Behrendt G, Johansson P, Tripathi P, Costa MR, Bek S, Heinrich C, Tiedt S, Colak D, Dichgans M, Fischer I, Plesnila N, Staufenbiel M, Haass C, Snapyan M, Saghatelyan A, Tsai LH, Fischer A, Grobe K, Dimou L, Gotz M. 2013. Reactive Glia in the Injured Brain Acquire Stem Cell Properties in Response to Sonic Hedgehog. Cell Stem Cell 12:426–439. [DOI] [PubMed] [Google Scholar]
- Smith MP, Cass WA. 2007. GDNF reduces oxidative stress in a 6-hydroxydopamine model of Parkinson’s disease. Neuroscience Letters 412:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Lu TY, Chai H, Xu J, Huang BS, Golshani P, Coppola G, Khakh BS. 2016. New Transgenic Mouse Lines for Selectively Targeting Astrocytes and Studying Calcium Signals in Astrocyte Processes In Situ and In-áVivo. Neuron 92:1181–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Niu W, Liu ML, Zou Y, Zhang CL. 2014. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun 5, 3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. 2014. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab 34:1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TH, Chen SL, Chiang YH, Lin SZ, Ma HI, Kuo SW, Tsao YP. 2000. Recombinant Adeno-Associated Virus Vector Expressing Glial Cell Line-Derived Neurotrophic Factor Reduces Ischemia-Induced Damage. Experimental Neurology 166:266–275. [DOI] [PubMed] [Google Scholar]
- Uesaka T, Nagashimada M, Enomoto H. 2013. GDNF Signaling Levels Control Migration and Neuronal Differentiation of Enteric Ganglion Precursors. The Journal of Neuroscience 33:16372–16382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV. 2009. Reactive Astrocytes Form Scar-Like Perivascular Barriers to Leukocytes during Adaptive Immune Inflammation of the CNS. J Neurosci 29:11511–11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang Q, Bao R, Zhang N, Wang Y, Polo-Parada L, Tarim A, Alemifar A, Han X, Wilkins HM, Swerdlow RH, Wang X, Ding S. 2017. Deletion of Nampt in Projection Neurons of Adult Mice Leads to Motor Dysfunction, Neurodegeneration, and Death. Cell Reports 20:2184–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lin SZ, Chiou AL, Williams LR, Hoffer BJ. 1997. Glial Cell Line-Derived Neurotrophic Factor Protects against Ischemia-Induced Injury in the Cerebral Cortex. The Journal of Neuroscience 17:4341–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Gw, Wu Gc, Cao Xd. 2000. Dynamic expression of glial cell line derived neurotrophic factor after cerebral ischemia. Neuroreport 11. [DOI] [PubMed] [Google Scholar]
- Winchenbach J, Dnking T, Berghoff SA, Stumpf SK, Hnlsmann S, Nave KA, Saher GP. (2016) Inducible targeting of CNS astrocytes in Aldh1l1-CreERT2 BAC transgenic mice. F1000Res 5, 2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship IR, Murphy TH. 2008. In Vivo Calcium Imaging Reveals Functional Rewiring of Single Somatosensory Neurons after Stroke. J Neurosci 28:6592–6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wang T, Sun GY, Ding S. 2010. Specific disruption of astrocytic Ca2+ signaling pathway in vivo by adeno-associated viral transduction. Neuroscience 170:992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T, Jikihara I, Fukumura M, Watabe K, Ohashi T, Eto Y, Hara M, Maeda M. 2000. Rescue of ischemic brain injury by adenoviral gene transfer of glial cell line-derived neurotrophic factor after transient global ischemia in gerbils. Brain Research 885:273–282. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Hakata K, Maeda A, Mochizuki C, Matsufuji H, Chino M, Yamori Y. 2007. Adenosine induces expression of glial cell line-derived neurotrophic factor (GDNF) in primary rat astrocytes. Neuroscience Research 59:467–474. [DOI] [PubMed] [Google Scholar]
- Yan M, Dai H, Ding T, Dai A, Zhang F, Yu L, Chen G, Chen Z. 2011. Effects of dexmedetomidine on the release of glial cell line-derived neurotrophic factor from rat astrocyte cells. Neurochemistry International 58:549–557. [DOI] [PubMed] [Google Scholar]
- Young CC, van der Harg JM, Lewis NJ, Brooks KJ, Buchan AM, Szele FG. 2013. Ependymal Ciliary Dysfunction and Reactive Astrocytosis in a Reorganized Subventricular Zone after Stroke. cercor 23:647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Xie Y, Wang T, Bi J, Li H, Zhang LQ, Ye SQ, Ding S. 2010. Neuronal protective role of PBEF in a mouse model of cerebral ischemia. J Cereb Blood Flow Metab 30:1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. 2014. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. The Journal of Neuroscience 34:11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Rempe D. 2010. Targeting astrocytes for stroke therapy. Neurotherapeutics 7:439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB, Barres BA. 2015. Glia in mammalian development and disease. Development 142:3805–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. GLAST-CreERT2-mediated gene recombination. A-D) Fluorescent images of s100b and tdTomato in the cortex (A-B) and hippocampus (C-D). The bottom panels are the high resolution images of the upper panels. Four-week old GLAST-CreERT2:GCaMP5-IRES-tdTomatof/f mice were injected with TAM (100 mg/Kg) for 5 consecutive days and sacrificed 2 weeks later.
Figure S2. Increased Cre activity in GLAST-CreERT2 mice after FIS. Fluorescent images of immunostaining of Cre and GFAP in the non-ischemic (A-B) and ischemic (C-D) sides of brain. Cre expression in the cortex of ischemic side were largely increased in GLAST-CreERT2 mice two days after PT. Notice many GFAP+ RAs are colocalized with Cre in the penumbra in ischemic side, and there are some Cre+ cells in non-ischemic side, but the GFAP expression is low.


