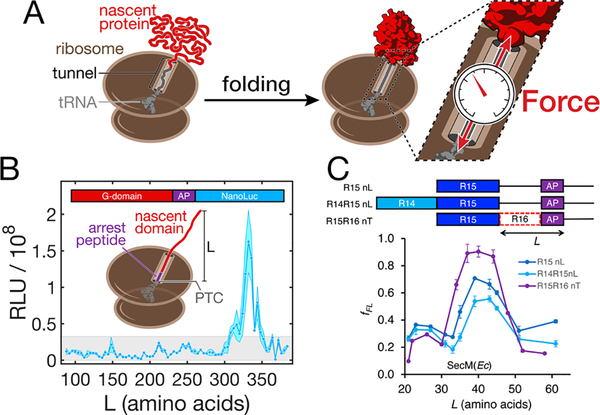
FIGURE 3.
Nascent chain folding generates mechanical force. (A) Folding of a nascent chain in close proximity to the ribosome sterically excludes it from the exit tunnel. When folding occurs close to the surface of the ribosome, it generates a mechanical force on the unfolded segment of the nascent chain within the tunnel. The force is experienced by and destabilizes the folded domain. It also acts on the C-terminal attachment point. Without stable nascent chain-tunnel interaction, the force propagates to the peptidyl transferase center, where the nascent chain is anchored by a tRNA, potentially affecting ribosome activity. (B) Steric force resulting from nascent protein folding is sufficient to release arrest peptide (AP)-mediated stalling of elongation. Coupling of arrest release to the synthesis of a reporter (NanoLuc luciferase) allows detection of nascent chain folding waypoints in live bacteria. This assay reveals that the main folding event during synthesis of the G-domain of EF-G occurs only after the complete domain (293 amino acids) has been fully extruded from the ribosome, around L = 332 amino acids. Stable intermediates are not detected in shorter chains. RLU: relative light units. From ref.[53](C) The arrest peptide assay also reports on nascent chain folding in vitro, shown here for the R15 domain of spectrin. The signal obtained for the R15 domain without neighboring domains (R15nL) is affected weakly if the upstream R14 domain is present (R14R15nL). However, presence of part of the downstream R16 domain (R15R16nT) shifts the onset and/or magnitude of the signal, indicating that the onset of R15 folding is sensitive to the presence of the N-terminus of R16. fFL: fraction full-length. Note that the length L is defined differently in panels B and C. From ref.[60], with permission