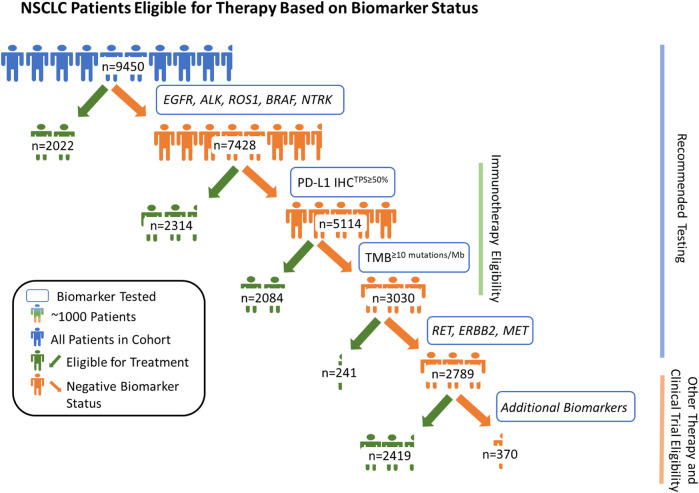
FIGURE 1.
Patients with non-small cell lung cancer (NSCLC) eligible for therapy based on biomarker status. By assessing genomic driver alterations, tumor mutational burden (≥10 mutations/Mb cut-off), and PD-L1 expression (TPS ≥ 50% cut-off), we show that CGP + PD-L1 IHC yielded potentially actionable results, per National Comprehensive Cancer Network (NCCN) guidelines, for 70.5% of the 9,450 patients with NSCLC. Among the remaining 29.5% (2,789/9,450) of patients, 86.7% (2,419/2,789) were potentially eligible for another biomarker-associated therapy and/or clinical trial based on their genomic profile. In total, combined CGP and PD-L1 IHC testing provided positive biomarker statuses for 96.1% of 9,450 patients with NSCLC when considering potential eligibility for biomarker associated therapies and clinical trial enrollment.