Figure 1. Participants with Reduction in Heavy Menstrual Bleeding.
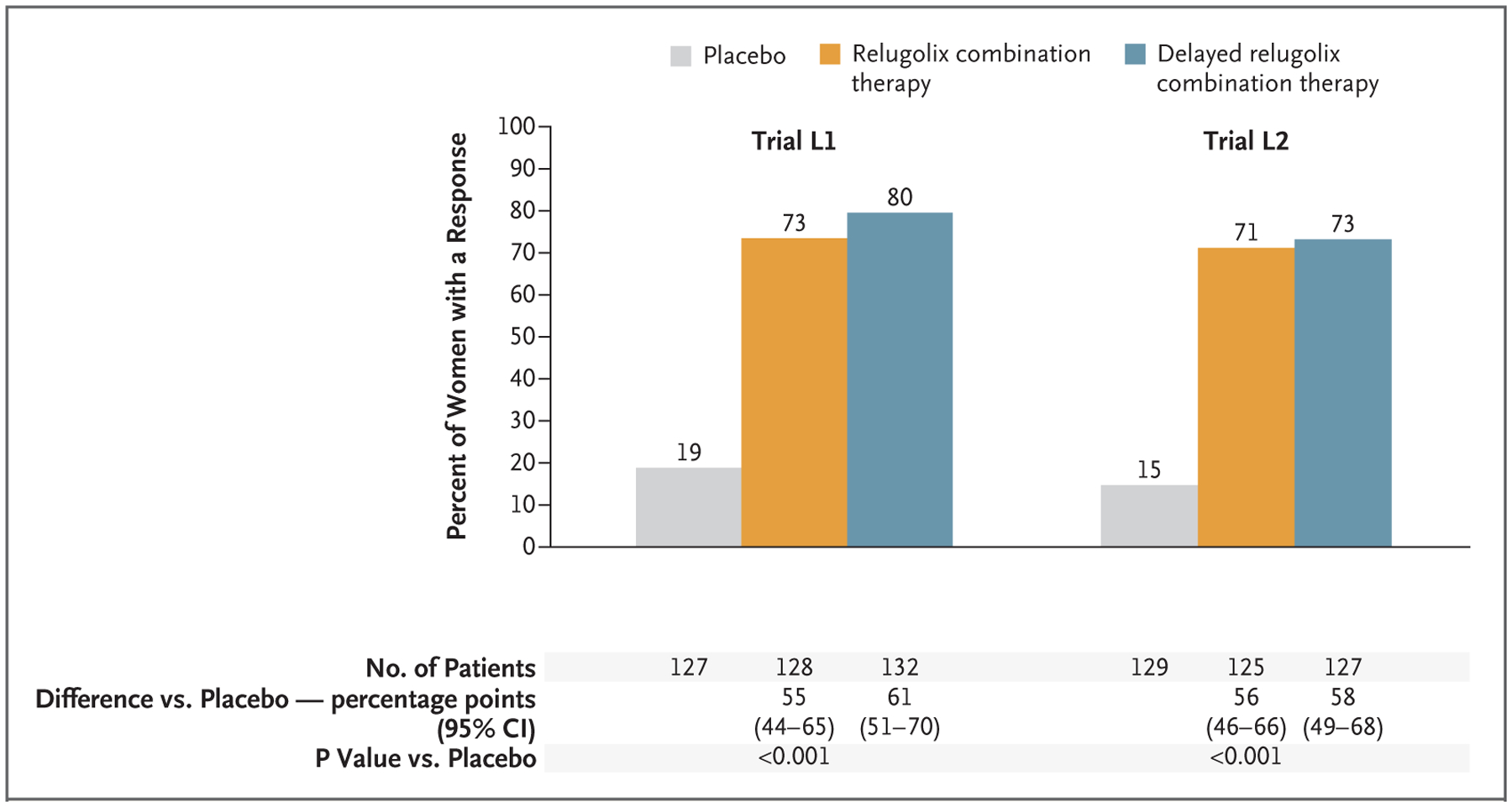
Shown are the percentages of women who had a response, which was defined as a volume of menstrual blood loss of less than 80 ml and a reduction of at least 50% from the baseline volume of menstrual blood loss, as measured by the alkaline hematin method, over the last 35 days of the treatment period. The primary end-point analysis in each trial was the comparison of relugolix combination therapy with placebo. CI denotes confidence interval, L1 LIBERTY 1, and L2 LIBERTY 2.
