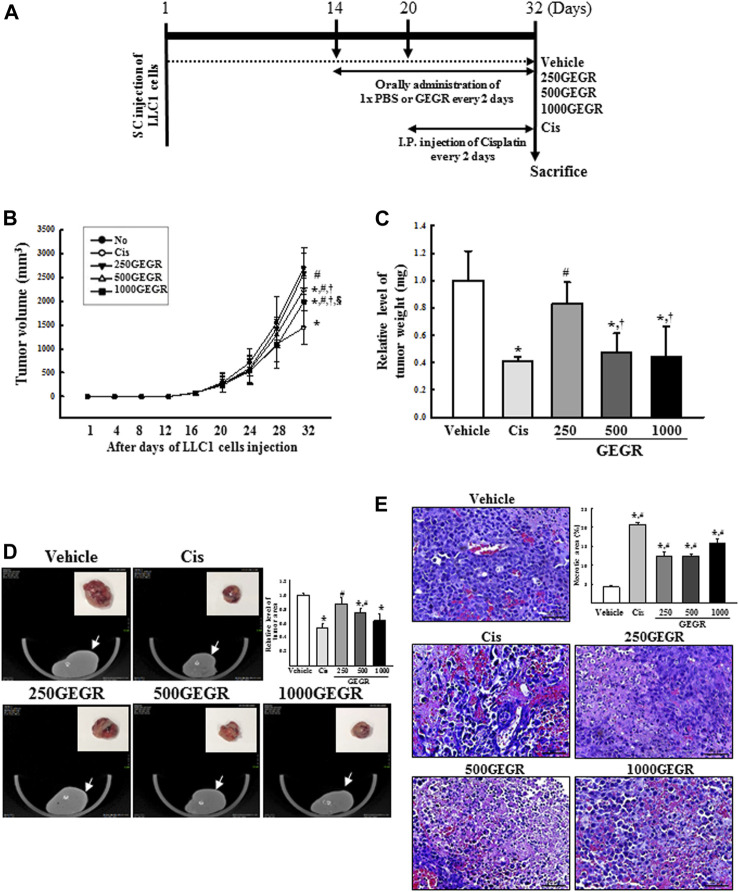
FIGURE 7.
Growth and histological structure of LLC1 tumor. (A) Experimental scheme for GEGR treatment. After subcutaneous injection of LLC1 cells, the syngeneic model was administered either of the three GEGR doses (250, 500 and 1,000 mg/kg) orally, or cisplatin intraperitoneally, from day 14 to day 32 or from day 20 to day 32, respectively. (B) Volume of LLC1-induced tumor. The size of solid tumor formed in the syngeneic model was measured using a caliper from days 1–32. (C) Weight of the LLC1 tumor. At day 32, tumors were collected form the syngeneic model, and their weight was measured using an electrical balance. (D) Tumor image of microCT. At day 32, all tumors were observed with microCT (Nano Focus Ray). Arrow indicates the tumor region, and actual tumor image is presented in the right corner. (E) Histopathological structure of tumor. Tumorigenic changes such as apoptosis, cyst, hemorrhage and angiogenesis were detected in H&E stained tumor sections at 400 × magnification. Five to six mice per group were used for microCT imaging, assessment of tumor volume, and preparation of tissue sections; H&E staining was analyzed in duplicate in each tumor. Data are reported as the means ± SD. *, p < 0.05 compared to the Vehicle treated group. #, p < 0.05 compared to the Cis treated group. †, p < 0.05 compared to the 250GEGR treated group. §, p < 0.05 compared to the 500GEGR treated group.