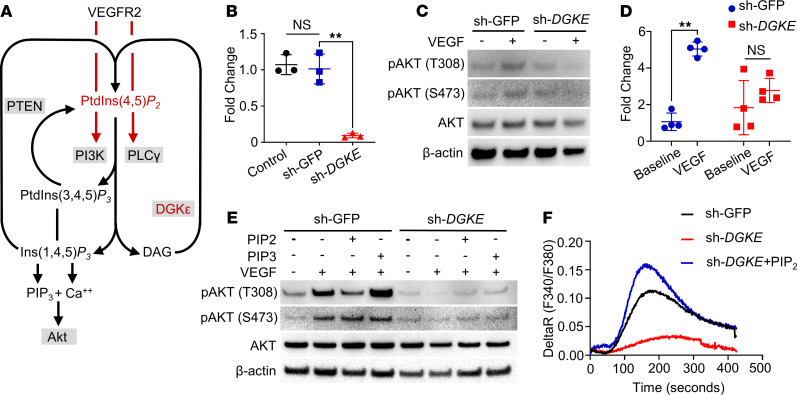
Figure 1. VEGFR2-dependent Akt activation is compromised in DGKE-knockdown human umbilical vein epithelial cells.
(A) Schematic representation of the phosphoinositide cycle and of some of the enzymes involved in the cycle (boxes). Red arrows point to the major enzymes (PI3K and phospholipase C γ, PLCγ) activated downstream of VEGFR2. Black arrows represent generation of PtdIns(1,4,5) P3 (PIP3) and Ca2+ downstream of PI3K and PLCγ, respectively, and activation of Akt. The common substrate of PI3K and PLCγ, PIP2, required for Akt activation is highlighted in red. DGKε is highlighted in red. (B) Efficiency of the sh-RNA knockdown in HUVECs measured by quantitative PCR (Q-PCR) compared with nontargeted control (sh-GFP) cells. Control: nontransfected cells. (C) Western blot showing impaired Akt activation (phosphorylation of threonine 308 and serine 473) in the shRNA-knockdown HUVECs upon VEGFA stimulation, compared with nontargeted control cells. (D) Expression of Cox2, measured by Q-PCR, is not induced in DGKE-knockdown HUVECs compared with nontargeted control cells stimulated with VEGFA. (E) Western blots showing that impaired Akt activation in the sh-RNA knockdown HUVECs upon VEGFA stimulation is partially reversed by PIP2 and PIP3 supplementation. (F) Changes in intracellular Ca2+ concentrations after VEGFA supplementation in DGKE-knockdown HUVECs, in DGKE-knockdown HUVECs after PIP2 supplementation, and in nontargeted HUVEC controls over time, measured by Fura-2 AM fluorescence. Data are from 3–4 independent experiments and are presented as mean ± SD. **: P < 0.01 by 1-way ANOVA in B and by Student’s t test in D. Each data point represents 1 experiment. PTEN, phosphatase and tensin homolog; pAKT, phosphorylated Akt; DeltaR: fluorescence ratio as 340 nm/380 nm.