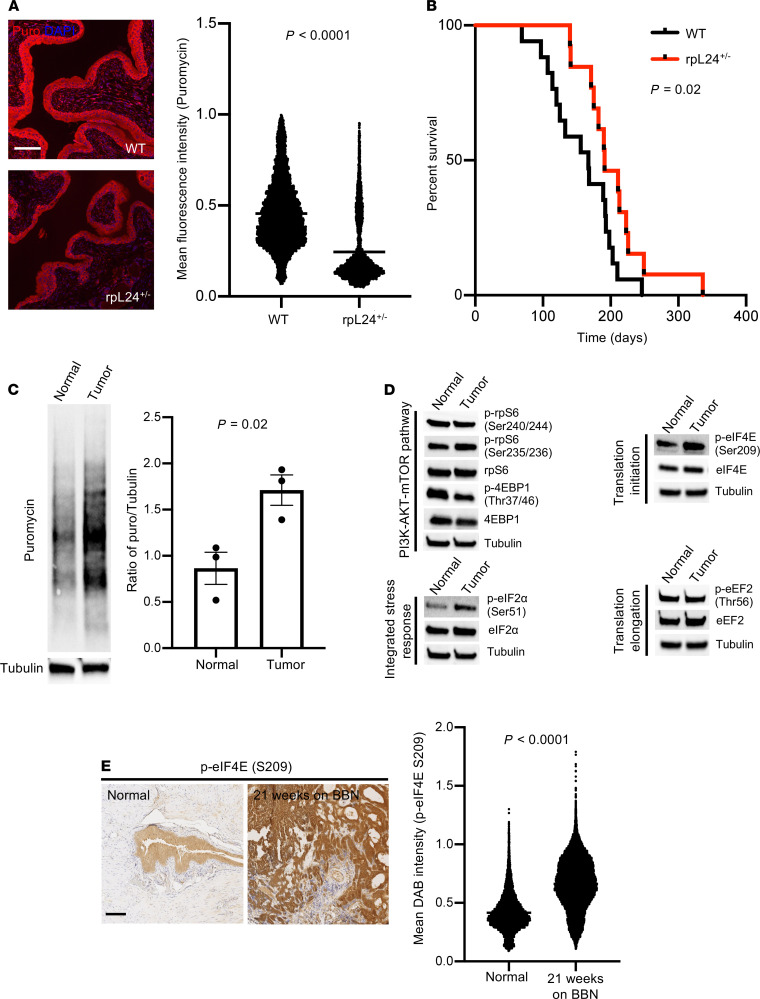
Figure 1. Optimal protein synthesis is necessary for efficient urothelial cell transformation in vivo, and eIF4E phosphorylation selectively increases in the context of bladder cancer formation.
(A) Puromycin incorporation in WT and rpL24+/– urothelium. Representative IF images show less protein synthesis in rpL24+/– mice compared with WT counterparts. Quantification of > 5000 cells/genotype (WT [n = 3], rpL24+/– [n = 2], P < 0.0001, t test). (B) Kaplan-Meier survival analysis of WT (n = 17) and rpL24+/– (n = 13) mice treated with 0.075% BBN ad libitum (P = 0.02, log-rank test). (C) Puromycin incorporation in normal and tumor organoids developed from WT and WT + BBN–treated mice. Representative puromycin Western blot. Quantification of n = 3 biological replicates (P = 0.02, t test). (D) Candidate gene analysis of translation regulators by Western blot using normal and BBN tumor organoids (n = 3 biological replicates). The same tubulin blot is used in the PI3K-AKT-mTOR pathway and integrated stress response figures. The same tubulin blot is used in the translation initiation and translation elongation figures. (E) eIF4E S209 phosphorylation in WT and BBN-treated C57BL/6 mice. Representative eIF4E S209 IHC. Quantification of > 5000 cells/genotype (Normal [n = 2], 21 weeks on BBN [n = 2], P < 0.0001, t test). Scale bars: 100 μm. Data are presented as mean ± SEM. See complete unedited blots in the supplemental material.