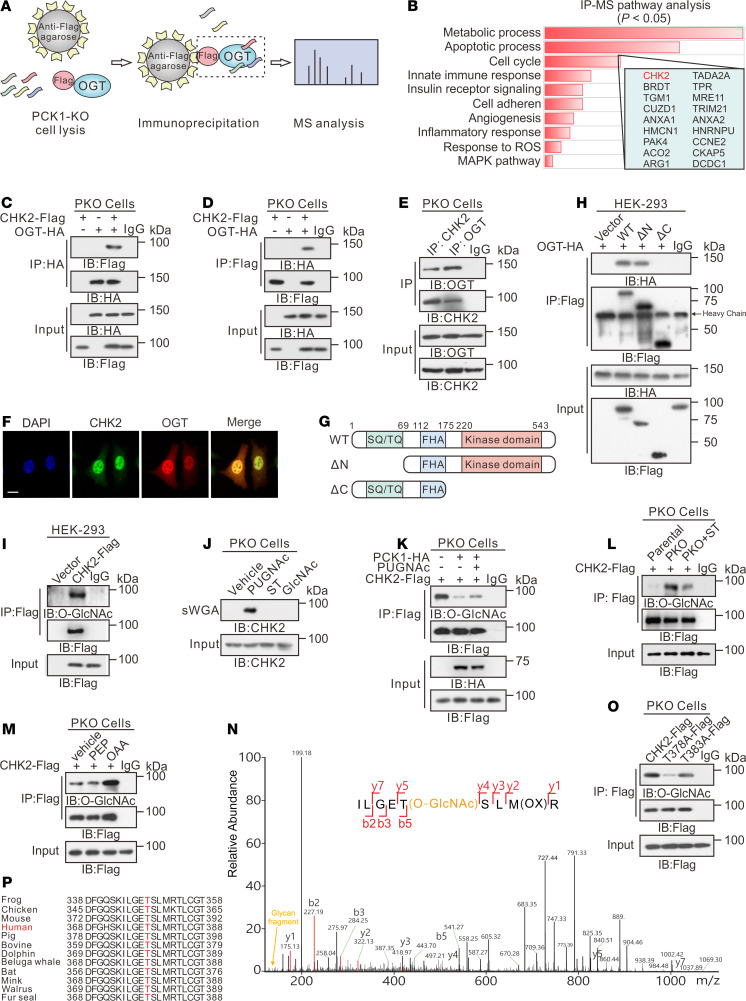
Figure 4. PCK1 deficiency promotes CHK2 O-GlcNAcylation at T378.
IP-LC-MS/MS analysis of O-GlcNAc-modified proteins, represented by (A) flowchart describing the processes used for IP-LC-MS/MS analysis, and (B) Kyoto Encyclopedia of Genes and Genomes–based analysis of significantly enriched pathways represented by proteins that bound to Flag-tagged OGT. Co-IP of OGT-HA and CHK2-Flag was examined using an anti-HA antibody (C) or an anti-Flag antibody (D) in PKO cells. (E) Co-IP of endogenous OGT and CHK2 in PKO cells. (F) Subcellular colocalization of OGT and CHK2 in SK-Hep1 cell was determined with immunofluorescence staining. Scale bar: 50 μm. (G) Schematic representation of the CHK2 constructs. WT CHK2 contains 3 domains, including a SQ/TQ cluster domain, a Forkhead-associated (FHA) domain, and a kinase domain. Truncation mutants of CHK2, comprising amino acids (aa) 69–543 or 1–221, were designated as ΔN and ΔC, respectively. (H) Interactions between OGT and full-length WT, the ΔN, or the ΔC in HEK293 cells were determined by co-IP. (I) CHK2 IP with anti-Flag M2 agarose beads in HEK293 cells transfected with CHK2-Flag or a vector control. (J) PKO cells were treated with 50 μM PUGNAc or 50 μM ST for 24 hours, incubated in 5 mM glucose, and followed by a sWGA pull-down assay. Western blot was determined by anti-CHK2. Cell lysates of PCK1-OE cells (K), PKO cells (L), or SK-Hep1 cells treated with 1 mM PEP or OAA (M) were immunoprecipitated with anti-Flag agarose beads and immunoblotted, as indicated. (N) LC-MS analysis of CHK2-Flag identified residue T378 as the CHK2 O-GlcNAcylation site, which corresponded to the O-GlcNAcylated CHK2 peptide ILGETSLMR. (O) IP with anti-Flag M2 agarose beads in PKO cells. Cells were transfected with vectors encoding Flag-tagged versions of WT CHK2, T378A CHK2, or T383A CHK2. (P) Cross-species sequence alignment of CHK2.