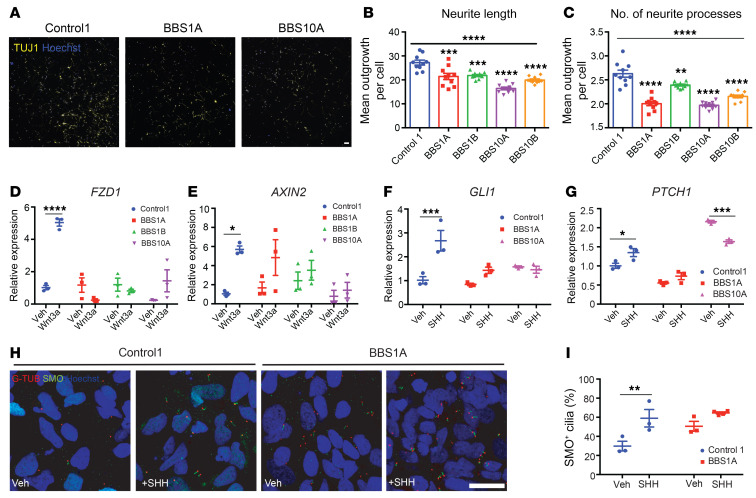
Figure 3. BBS mutations disturb neurite outgrowth and impair Wnt and SHH signaling in TUJ1+ iPSC–derived neurons.
(A–C) Neurite outgrowth assay of control and BBS iPSC–derived neurons on day 30 of differentiation. (A) TUJ1 staining of control and BBS mutant cultures. Scale bar: 50 μm. Mean neurite length (B) and average number of neurite processes (C) were calculated using the neurite outgrowth tool in MetaMorph software based on TUJ1 and Hoechst staining (n = 10 independent wells, 2,500 cells/well). **P < 0.01, ***P < 0.001, ****P < 0.0001 by 1-way ANOVA followed by Bonferroni’s multiple-comparison test (vs. control 1); the asterisks above the horizontal bars in B and C are for the 1-way ANOVA that permitted the pair-wise testing. (D and E) Wnt signaling is impaired in BBS iPSC–derived neurons. Control 1, BBS1A, BBS1B, and BBS10A iPSC–derived neurons (day 30) were treated with vehicle (Veh) or 100 ng/mL Wnt3a for 16 hours. Frizzled 1 (FZD1) and AXIN2 mRNA levels were determined by qPCR (n = 3). (F and G) SHH signaling is reduced in BBS iPSC–derived neurons. Control and BBS iPSC–derived neurons (day 30) were treated with vehicle or 100 ng/mL SHH for 16 hours. GLI1 and PTCH1 mRNA levels were analyzed by qPCR (n = 3). (H) Smoothened (SMO) staining in SHH-treated control and BBS iPSC–derived neurons. Control and BBS1A iPSC–derived neurons were treated with vehicle or 100 ng/mL SHH overnight. Neurons were fixed and stained with anti-SMO and anti–γ-tubulin (G-TUB, basal body) antibodies for cilia and Hoechst for nuclei. Scale bar: 20μm. (I) Quantification of SMO+ cilia in E. Hoechst was used as nuclear marker. SMO+ cilia percentage was calculated by (SMO+ cells/Hoechst+ cells) × 100 (n = 3 independent images). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by 2-way ANOVA followed by Bonferroni’s multiple-comparison test (D–G and I).