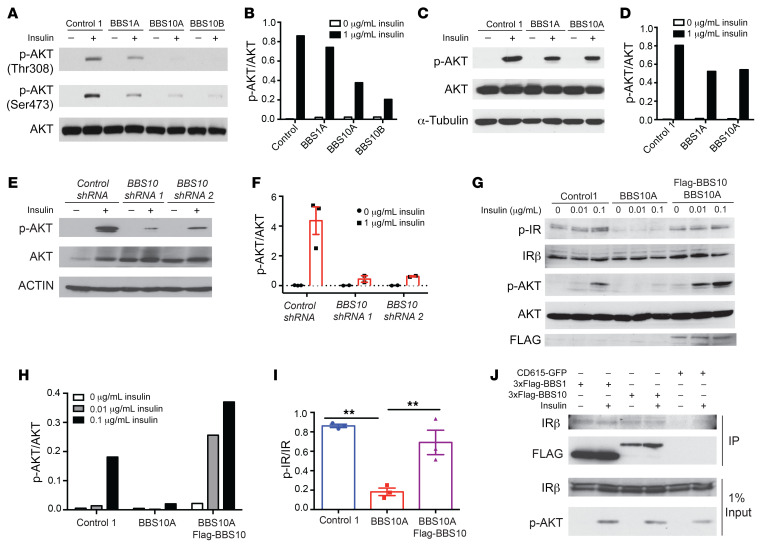
Figure 4. BBS1 and BBS10 bind to the insulin receptor and influence insulin signaling.
(A) BBS mutations disrupt insulin signaling in human fibroblasts. Western blot (WB) analysis of insulin signaling as indicated by phosphorylation of AKT in control and BBS fibroblasts. Fibroblasts were serum starved overnight and treated with 0 or 1 μg/mL insulin for 30 minutes. AKT, p-AKT Thr308, and p-AKT Ser473 were probed. (B) Quantification of p-AKT (Thr308)/AKT from A (n = 1). (C) BBS mutations abrogate insulin signaling in iPSC-derived TUJ1+ neurons. Control, BBS1A, and BBS10A iPSC–derived neurons (day 30) were serum starved overnight and treated with 0 or 1 μg/mL insulin for 30 minutes. p-AKT Ser473, AKT, and α-tubulin (A-TUB) were probed. (D) Quantification of p-AKT/AKT from WB in C (n = 1). (E) Diminished insulin signaling in neurons derived from 2 BBS10 shRNA-knocked-down iPSC lines as indicated. iPSC-derived TUJ1+ neurons (day 30) were serum starved overnight and treated with 0 or 1 μg/mL insulin for 30 minutes. p-AKT Ser473, AKT, and actin were examined by WB. (F) Quantification of p-AKT/AKT from WB in E (n = 2–3). (G) BBS10 disrupts insulin signaling by disturbing phosphorylation of the insulin receptor (IR) in day 12 neuronal progenitors (NPs). WB analysis of insulin signaling molecules as indicated in control, BBS10A, and BBS10A-FLAG-BBS10 transgenic NPs after 30-minute treatment with 0.01 and 0.1 μg/mL insulin. FLAG was used to confirm the overexpression of WT BBS10. (H and I) Quantification of p-AKT/AKT (H) (n = 1) and p-IR/IR (I) (n = 3) in D. **P < 0.01 by 1-way ANOVA followed by Tukey’s multiple-comparison test. (J) Coimmunoprecipitation (IP) confirms the interaction between BBS proteins and the IR. 293FT cells were transfected with CD615-GFP or CD615-3×FLAG-BBS1-GFP or CD615-3×FLAG-BBS10-GFP for 48 hours before insulin (1 μg/mL) treatment (30 minutes). IRβ, p-AKT Ser473, and FLAG were probed in FLAG IP samples and total input.