Fig. 1. Overview of the atmospheric acidity, nutrient, and trace element cycles.
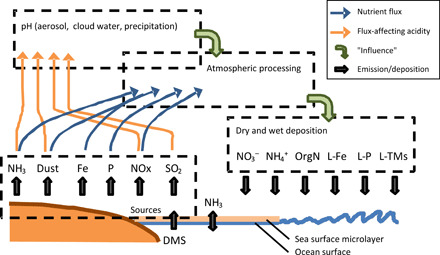
Emissions of NH3, NOx, SO2, and dust influence atmospheric acidity (orange arrows). Dust, anthropogenic trace element emissions (abbreviated as Fe), and anthropogenic and biological sources of P, NH3, and NOx contribute to the atmospheric nutrient/trace element burden (blue arrows). The majority of sources are terrestrial, although ship-based emissions of Fe and NOx are important and marine emissions of dimethyl sulfide (DMS) are a substantial source of SO2, particularly in the Southern Hemisphere. Acidity-driven atmospheric processing alters the labile nutrient flux to the ocean, either by affecting the gas-aerosol partitioning or by altering the labile fractions of Fe (L-Fe), P (L-P), and trace metals (L-TM). Organic nitrogen compounds (OrgN) are also generated during atmospheric processing but are not discussed here.
