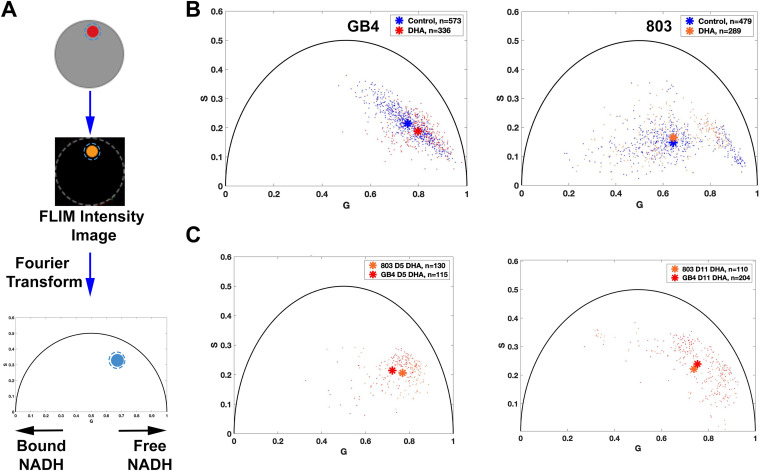
FIG 4.
Metabolic phenotyping of untreated control versus DHA-treated GB4 and 803 parasites. (A) Schematic flow of FLIM-phasor data collection and analysis. Parasites were stained only with MT, sorted by FACS, and seeded into a 10- by 10-μm polydimethylsiloxane (PDMS) stencil microwell covered with 0.01% poly-lysine. The region of interest (ROI) in a parasite is established by the boundary of MT fluorescence, and this ROI mask is applied to the lifetime datafile represented by its FLIM intensity image. At least 10 million photon events are counted while limiting the time of exposure of these parasites to 750 nm excitation. Next, Fourier transformation of the FLIM data is performed on the data from each pixel, and the frequency domain results are averaged to represent a data point on the 2D phasor plot. Each position has coordinates G (from 0 to 1) and S (from 0 to 0.5); single exponential decays fall upon the semicircle, whereas complex multiexponential decays, such as occur with fluorescent lifetimes of metabolic coenzymes, fall within the semicircle. A shift to the right (shorter fluorescence lifetime) indicates increased free NADH, whereas a shift to the left (longer lifetime) indicates increased enzyme-bound NADH. (B) Autofluorescence FLIM-phasor graphs from the GB4 and 803 parasite populations at t = 50 h. The phasor distribution of untreated control GB4 parasites has a mean coordinate of 0.7542, 0.2150, whereas the distribution of DHA-treated parasites has a mean coordinate of 0.7971, 0.1879 (P < 0.0001, Pillai trace). This shift after DHA treatment is toward increased free NADH, consistent with the quiescent metabolic state of persisters. The distribution of untreated 803 parasites at t = 50 h (right) has a mean coordinate of 0.6341, 0.1400, and the phasor distribution of DHA-treated parasites has a mean coordinate of 0.6270, 0.1570. The distribution of DHA-treated 803 parasites is slightly shifted upward relative to control (P < 0.05, Pillai trace) but not to the left or right, consistent with the presence of actively replicating 803 parasites that survived ring-stage exposure to DHA. Asterisks mark the mean phasor positions of the control and DHA-treated parasite distributions. Comparison of intensity images in the same field of view before and after FLIM acquisition confirmed little or no photobleaching. (C) Autofluorescence FLIM-phasor graphs for DHA-treated/sorbitol-selected GB4 and 803 on day 5 and day 11. In the phasor distributions from day 5 (left), the treated GB4 and 803 parasites have mean coordinates of 0.72, 0.21 and 0.77, 0.21, respectively. Consistent with depletion of the many actively replicating parasites from the 803 population by three daily sorbitol treatments, the 803 phasor distribution is shifted right with quiescence and increased levels of free NADH in persisters. The genetic backgrounds and baseline metabolic states of African GB4 and Southeast Asian 803 parasites may contribute to the different positions of the 803 and GB4 coordinates (P < 0.0001, Pillai trace).