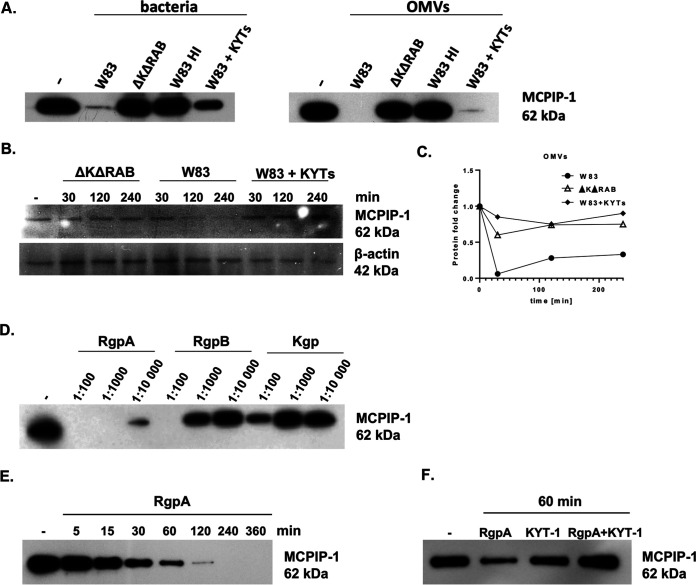
FIG 2.
Proteolysis of MCPIP-1 depends on gingipain activity. (A) Recombinant MCPIP-1 protein was incubated with the P. gingivalis wild-type W83 strain, the gingipain-deficient ΔKΔRAB strain, or outer membrane vesicles (OMVs) isolated from both species for 1 h at 37°C. Wild-type bacteria and OMVs were heat-inactivated (HI) at 90°C for 20 min or inactivated with the gingipain-specific inhibitors KYT-1 and KYT-36 (KYTs) for 20 min. (B) Western blot analysis of protein lysates of gingival keratinocytes (TIGKs) infected with P. gingivalis W83 or ΔKΔRAB strains (multiplicity of infection [MOI], 1:100). Gingipain activity was inhibited using a mixture of KYT-1 and KYT-36 inhibitors. (C) Time-dependent MCPIP-1 protein degradation in TIGKs estimated by Western blotting, quantified by densitometry, and presented as a fold change normalized to β-actin levels. Cells were incubated with OMVs with or without KYTs. (D) Recombinant MCPIP-1 protein was incubated for 60 min at 37°C with purified gingipains RgpA, RgpB, or Kgp at indicated molar ratios (gingipain:MCPIP-1) and (E) with RgpA at a 1:10,000 molar ratio for the indicated times. (F) Determination of MCPIP-1 level after exposure of recombinant protein to active RgpA or RgpA inactivated by KYT-1 at a molar ratio of 1:10,000. (A to F) Representative Western blot results are shown.