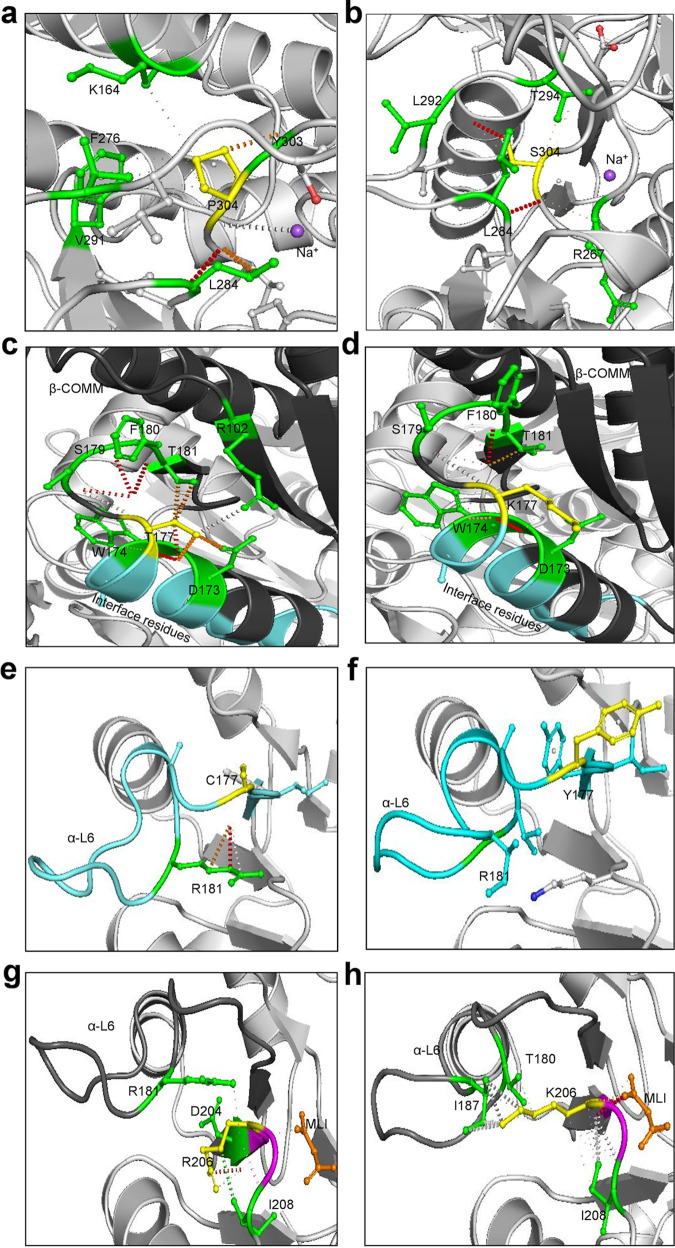
FIG 12.
Effect of wild-type and mutant residues on interatomic interactions in TrpA and TrpB protein structures and small-molecule ligand binding. (a and b) The wild-type residue P304 (yellow) of E_Bour β-subunit (a) and the mutant residue S304 (yellow) of E_SotonE8 β-subunit (b) interactions with neighboring residues (green) and the ligand Na+ (purple). (c and d) The wild-type residue T177 (yellow) of I_UW12 β-subunit (c) and the mutant residue K177 (yellow) of I_UK913341 β-subunit (d) interacting with αβ interface (cyan) and neighboring residues (green). (e and f) The wild-type residue C177 (yellow) of G_UW57 α-subunit (e) and mutant residue Y177 (yellow) of G_UK750369 α-subunit (f) interactions with residue R181 (green) located in the α-L6 (cyan). (g and h) The wild-type residue R206 (yellow) of G_UW57 α-subunit (g) and mutant residue K206 (yellow) of G_UK750369 α-subunit (h) interactions with residues (green) in the α-L6 and malonate ion (MLI; orange). The α-L6 region shown in charcoal gray and catalytic site in magenta. Polar contacts (red), weak polar contacts (orange), van der Waals clash interactions (gray), and hydrophobic van der Waals contact (green) between residues and ligands are shown as dotted lines.