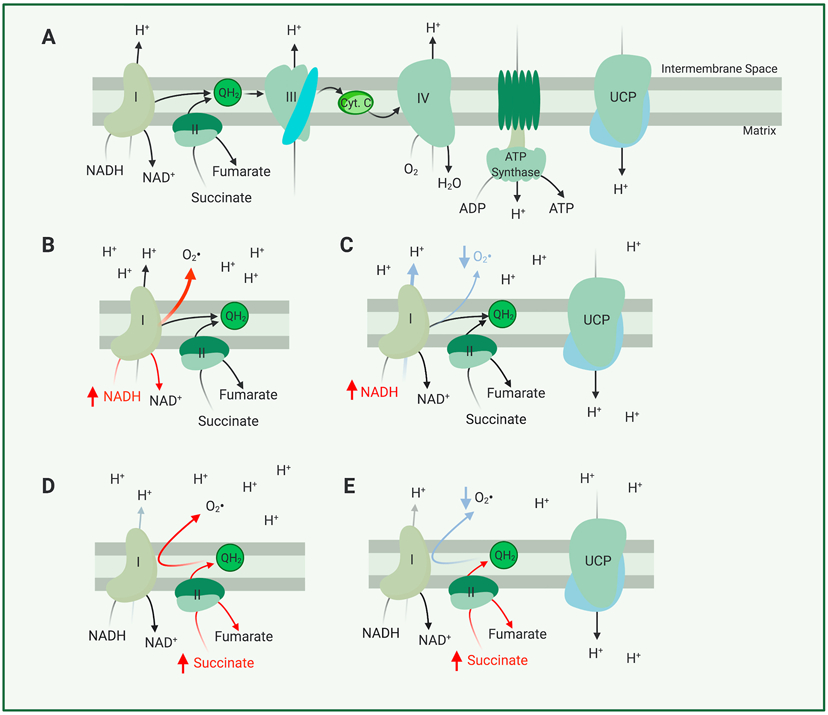
Figure 1. Main Components of the Electron Transport Chain and their Roles in Forming ROS.
(A) In this simplified model of the electron transport chain, electrons are either derived from oxidation of NADH by complex I (NADH:CoQ Oxidoreductase) or succinate by complex II (succinate dehydrogenase). These electrons are transported through complex III (Coenzyme Q: Cytochrome C reductase) and complex IV (Cytochrome C Oxidase) where they power translocation of protons from the mitochondrial matrix to the intermembrane space, before finally being used to reduce O2 to H2O at complex IV. (B) Should more NADH be supplied than complex I can quickly oxidize, electrons from NADH will interact with O2 to form a superoxide molecule (a form of ROS) (C) Uncoupling, mediated by a protonophore or an uncoupling protein, can decrease production of superoxide by NADH. (D) Should more succinate be supplied than complex III or IV can transport electrons from, electrons from succinate can be transported ‘in reverse’ to complex I through the shared complex I and complex II substrate coenzyme Q10. Reverse electron transport through complex I further aggravates superoxide production. (E) Uncoupling mediated by a protonophore or an uncoupling protein may allow complex I, III, or IV to transport electrons more quickly in the conventional ‘forward direction’, preventing reverse electron transport and thus aggravated superoxide production.