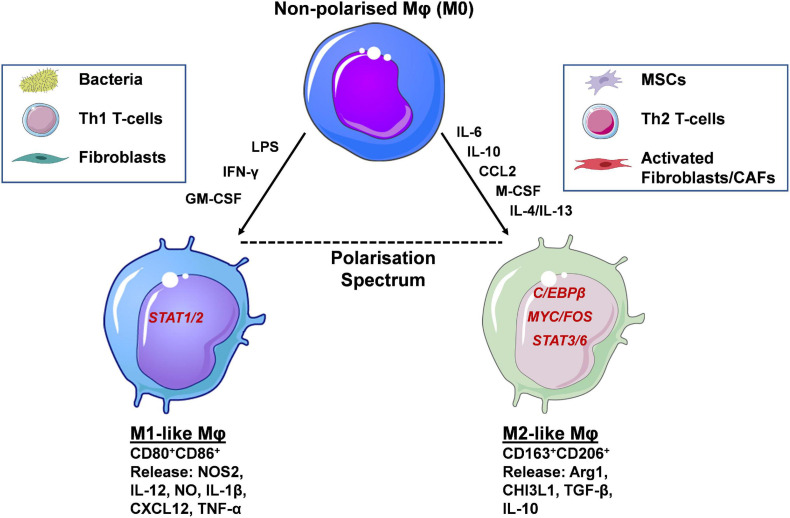
FIGURE 2.
Macrophage polarisation occurs via activation of distinct transcription factors and determines their function. Macrophages (Mφs) are highly plastic cells, adopting phenotypic and functional states that can be classified along a polarisation spectrum. At the two opposite ends of this spectrum lie the M1-like and M2-like Mφs. The former are induced by the presence of inflammatory mediators (e.g., LPS, IFN-γ, GM-CSF), signalling through STAT1/2, and display expression of CD80 and CD86 cell surface markers. Soluble, pro-inflammatory mediators released resemble Th1-like cytokines such as IL-12, IL-1β and TNF-α. Functional polarisation towards an M1-like phenotype occurs in the presence of bacteria, Th1 T-cells and fibroblasts and their respective secreted factors. At the other end of the spectrum lie alternatively activated M2-like Mφs, induced by IL-6, IL-10, M-CSF, CCL2, and IL-4/IL-13 released by mesenchymal stem/stromal cells (MSCs), Th2 T-cells and fibroblasts/cancer associated fibroblasts (CAFs). The main transcription factors involved in M2-like polarisation are C/EBPβ, MYC/FOS, and STAT3/6. Their secretome includes a number of anti-inflammatory, Th2-like mediators, such as Arg1, TGF-β and IL-10. Cell-surface expression of CD163 and CD206 distinguish M2-like from M1-like Mφs and have the potential to be used as prognostic markers in a number of human cancers, including AML (created using Servier Medical Art; Created using Biorender.com).