Abstract
Background
Data from the INMARK trial were used to investigate the feasibility and validity of home spirometry as a measure of lung function decline in patients with idiopathic pulmonary fibrosis (IPF).
Methods
Subjects with IPF and preserved forced vital capacity (FVC) were randomised to receive nintedanib or placebo for 12 weeks followed by open-label nintedanib for 40 weeks. Clinic spirometry was conducted at baseline and weeks 4, 8, 12, 16, 20, 24, 36 and 52. Subjects were asked to perform home spirometry at least once a week and ideally daily. Correlations between home- and clinic-measured FVC and rates of change in FVC were assessed using Pearson correlation coefficients.
Results
In total, 346 subjects were treated. Mean adherence to weekly home spirometry decreased over time but remained above 75% in every 4-week period. Over 52 weeks, mean adherence was 86%. Variability in change from baseline in FVC was greater when measured by home rather than clinic spirometry. Strong correlations were observed between home- and clinic-measured FVC at all time-points (r=0.72–0.84), but correlations between home- and clinic-measured rates of change in FVC were weak (r=0.26 for rate of decline in FVC over 52 weeks).
Conclusion
Home spirometry was a feasible and valid measure of lung function in patients with IPF and preserved FVC, but estimates of the rate of FVC decline obtained using home spirometry were poorly correlated with those based on clinic spirometry.
Short abstract
In a 52-week study in 346 subjects with idiopathic pulmonary fibrosis, mean adherence to weekly home spirometry was 86%. Estimates of the rate of decline in forced vital capacity obtained using home and clinic spirometry were poorly correlated. https://bit.ly/2WjIQ4b
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic fibrosing interstitial lung disease (ILD) characterised by decline in lung function [1]. Although IPF is always progressive, the rate and pattern of forced vital capacity (FVC) decline are variable among individuals [1–3]. Lung function has traditionally been measured periodically in a clinic-based setting, supervised by trained clinicians, but measurements obtained at home using a hand-held device have been shown to correlate well with clinic-based measurements over a 3–12-month period [4–8]. Home spirometry may offer advantages over clinic spirometry by increasing convenience for patients and providing more frequent measurements of lung function, enabling earlier detection of disease progression or acute exacerbations [4, 6, 9]. More frequent assessment of lung function via home spirometry might also provide improved analytical sensitivity, reducing the sample size required to power clinical trials [6]. However, in a recent trial conducted in subjects with unclassifiable ILD, the pre-specified analysis model could not be applied to the home spirometry measurements, in part due to issues with the reliability of the measurements [10]. More data are needed on the utility of home spirometry in the monitoring of lung function both in clinical trials and clinical practice.
In the INMARK trial in subjects with IPF and preserved lung function, lung function was assessed using both home and clinic spirometry over 52 weeks [11]. We used data from the INMARK trial to assess the feasibility and validity of home spirometry as a measure of lung function decline in subjects with IPF.
Methods
Study design and subjects
The primary objective of the INMARK trial was to investigate the effects of nintedanib on circulating biomarkers. The trial design has been described [11]. Briefly, subjects who had been diagnosed with IPF in the previous 3 years and had FVC ≥80% predicted were randomised 1:2 to receive nintedanib 150 mg twice daily or placebo for 12 weeks, followed by an open-label period in which all subjects received nintedanib 150 mg twice daily for 40 weeks [11]. Home spirometry devices (SpiroPro; ERT, Philadelphia, PA, USA) and instructions were given to subjects at screening. To be eligible for the trial, subjects were required to perform one or more home spirometry readings between screening and randomisation (a period of ≤28 days). The last measurement taken prior to the first intake of nintedanib or placebo was used as the baseline measurement.
Home and clinic spirometry
Subjects were asked to perform home spirometry (with three or more efforts) at least once a week, and ideally daily, throughout the trial. The highest value of the three or more efforts was recorded as the measurement. Subjects were asked to perform home spirometry in the morning, preferably between 08:00 and 11:00 h. An acoustic alarm on the device was activated daily at 09:00 and 09:30 h if the subject had not completed one or more efforts. For every measurement, the device showed the subject their highest value for FVC % predicted (calculated according to Quanjer et al. [12]) and informed them if they had experienced a relative decline in FVC ≥10% predicted from baseline; in this instance, subjects were advised to call their doctor. At each visit, subjects were re-trained on how to perform home spirometry if their adherence to weekly home spirometry since the last visit was <80% or as deemed necessary by the site. Adherence to weekly home spirometry was calculated as the number of weeks that a subject provided one or more measurements divided by the number of weeks that they were followed in the trial. Thus, 100% adherence was defined as provision of one or more measurements per week for all the weeks that the subject was in the trial.
Clinic spirometry was conducted at baseline and weeks 4, 8, 12, 16, 20, 24, 36 and 52. Clinic spirometry was centrally reviewed, and ongoing feedback and training were provided to the sites.
Analyses
Correlations between the following assessments at every time-point were assessed using the Pearson correlation coefficient (r): home and clinic measurements of FVC (mL), forced expiratory volume in 1 s (FEV1) (mL) and forced expiratory volume in 6 s (FEV6) (mL), home and clinic measurements of changes from baseline in FVC (mL), FEV1 (mL) and FEV6 (mL), and home and clinic measurements of rates of decline in FVC (mL), FEV1 (mL) and FEV6 (mL). In the analysis of correlations, the home measurement performed closest to the clinic visit was used (but the home spirometry device did not capture a measurement on the same day as a clinic visit).
The annual rates of decline in FVC and FEV6 were assessed using random coefficient piecewise regression with fixed effects for sex, age and height, and random effects of patient-specific intercept, time and a piecewise knot at week 12. Acute exacerbations, defined as in the INPULSIS trials [13], were reported by investigators using a tick box on the case report form and were not adjudicated. In subjects who had an investigator-reported acute exacerbation, all available home and clinic measurements of FVC (mL) before and after the acute exacerbation were plotted. Analyses were conducted using SAS (SAS Institute, Cary, NC, USA). Analyses were descriptive and exploratory.
Results
A total of 346 subjects were treated in the INMARK trial (116 randomised to nintedanib and 230 randomised to placebo). At baseline, mean±sd FVC was 3305±1060 mL and 99.6±23.8% predicted based on home spirometry and 3241±812 mL and 97.5±13.5% predicted based on clinic spirometry. In total, 83.5% of the subjects who were randomised completed 52 weeks of treatment.
Annual rate of decline in FVC and FEV6
In subjects treated with nintedanib for 52 weeks, the adjusted mean±se home- and clinic-measured rates of FVC decline were −127.2±76.3 and −88.8±23.9 mL·year−1, respectively, and the adjusted mean±se home- and clinic-measured rates of FEV6 decline were −112.6±69.5 and −90.5±22.3 mL·year−1, respectively. In subjects treated with placebo for 12 weeks followed by nintedanib for 40 weeks, the adjusted mean±se home- and clinic-measured rates of FVC decline were −111.8±54.7 and −104.1±17.0 mL·year−1, respectively, and the adjusted mean±se home- and clinic-measured rates of FEV6 decline were −131.8±49.9 and −103.9±15.9 mL·year−1, respectively.
Adherence to home spirometry
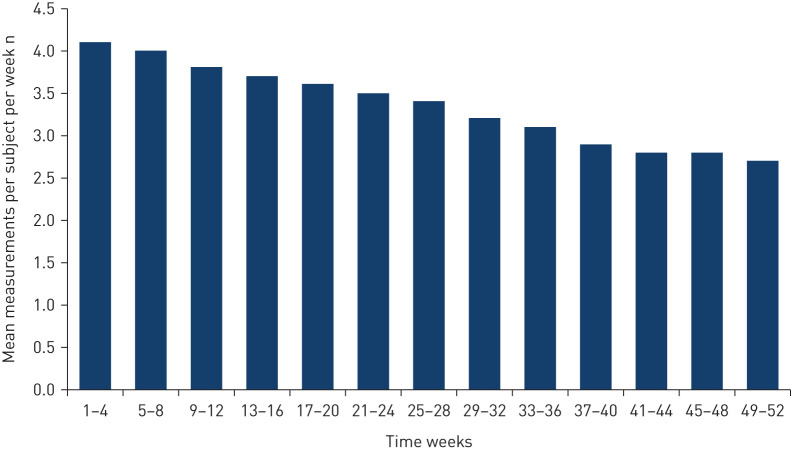
Over 52 weeks, the mean±sd number of home spirometry measurements per subject was 165±115 (table 1). The mean±sd number of measurements per subject per week was 3.4±2.6 and the median was 3.0. The mean number of measurements per subject per week decreased over the trial but remained above 2.5 in every 4-week period (figure 1).
TABLE 1.
Home spirometry measurements per subject over 52 weeks
| Nintedanib | Placebo/nintedanib# | All subjects | |
| Subjects | 116 | 230 | 346 |
| Home spirometry measurements | |||
| Mean±sd | 157±106 | 170±119 | 165±115 |
| Minimum | 3 | 3 | 3 |
| Median | 125 | 136 | 132 |
| Maximum | 362 | 633 | 633 |
Data are presented as n, unless otherwise stated. #: subjects received placebo (blinded) for 12 weeks followed by open-label nintedanib for 40 weeks.
FIGURE 1.
Mean number of home spirometry measurements per subject per week. Analysis based on the total number of home spirometry measurements collected and the number of subjects who were still followed in the trial within the time period.
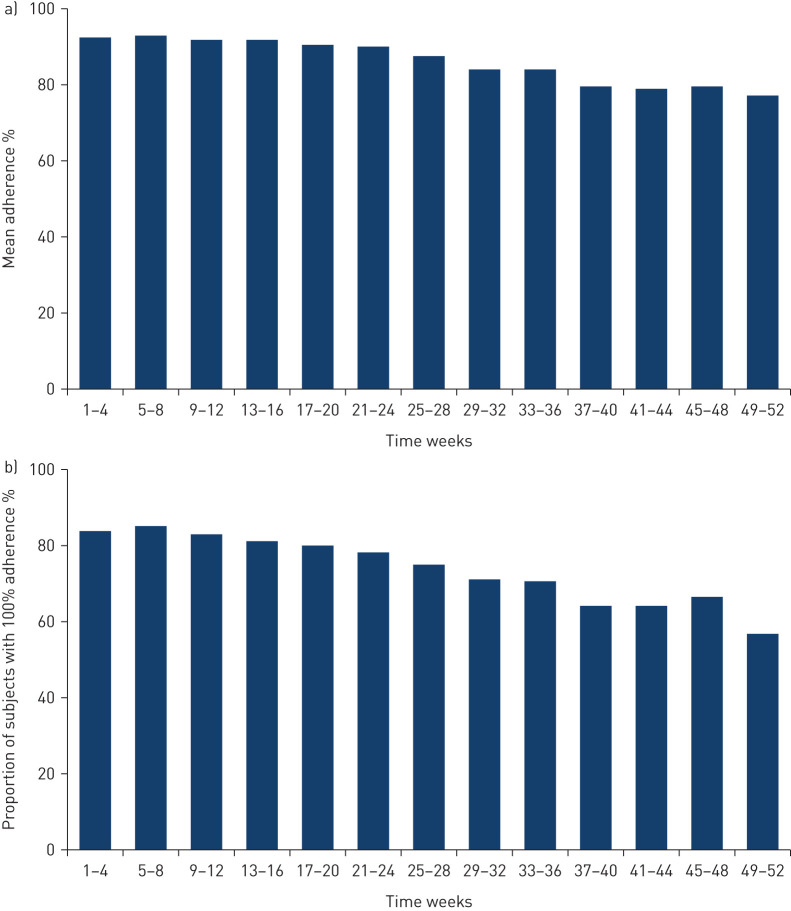
Over 52 weeks, mean and median adherence to weekly home spirometry were 86% and 96%. Mean adherence to weekly home spirometry decreased over the trial but remained above 75% in every 4-week period (figure 2a). The proportion of subjects with 100% adherence decreased over the trial but remained above 50% in every 4-week period (figure 2b and supplementary figure S1). Over 52 weeks, 31% of subjects had 100% adherence to weekly home spirometry.
FIGURE 2.
a) Mean adherence to weekly home spirometry. Adherence to weekly home spirometry was calculated as the number of weeks that a subject provided at least one measurement divided by the number of weeks that they were followed in the trial. b) Proportion of subjects with 100% adherence to weekly home spirometry. 100% adherence was defined as provision of at least one measurement per week for all the weeks that the subject was in the trial. The total number of subjects who were still followed in the trial within the time period was used as the denominator.
Subjects who had 100% adherence to weekly home spirometry (n=108) had slightly higher mean FVC and diffusing capacity of the lung for carbon monoxide at baseline than subjects who had <100% adherence (n=238) (supplementary table S1). Permanent discontinuation of trial medication was less common among subjects with 100% versus <100% adherence to weekly home spirometry (4.6% versus 21.8%).
Timing of home spirometry measurements
Over 52 weeks, 45.7% of subjects provided only one measurement on any day on which they provided a measurement. Most subjects took some of their measurements in the morning (defined as between 05:00 and 12:00 h) and some in the afternoon/evening (defined as between 12:00 and 05:00 h) (supplementary figures S2 and S3). The mean±sd FVC at baseline was similar between measurements taken in the morning and the afternoon/evening (3379±1062 and 3344±1277 mL, respectively). The mean FVC over time was variable, with greater variability in the measurements taken in the afternoon/evening than in the morning (supplementary figure S4).
Correlations between FVC and FEV6 measured using home and clinic spirometry
Correlations between FVC and FEV6, and changes in FVC and FEV6, measured using home and clinic spirometry are presented in figures 3a–c. Strong correlations were observed between home and clinic measurements of FVC (r=0.72–0.84), home and clinic measurements of FEV6 (r=0.71–0.85), and clinic measurements of FVC and home measurements of FEV6 (r=0.71–0.84) at all individual time-points (figure 3a). Correlations between home and clinic measurements of FVC were weaker in subjects who provided more than three versus three or less home spirometry measurements per week (r=0.63–0.75 versus r=0.78–0.94).
FIGURE 3.
“Heatmaps” depicting correlations between a) lung function variables measured at home and in clinic at different time-points (r≥0.5 for all correlations), b) changes from baseline in lung function variables measured at home and in clinic at different time-points, and c) rates of decline in lung function variables measured at home and in clinic at different time-points. FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; FEV6: forced expiratory volume in 6 s. Darker shades of red or blue indicate stronger positive or negative associations, respectively.
The variability in change from baseline in FVC was greater when measured using home spirometry than clinic spirometry (figure 4). Correlations between home- and clinic-measured changes from baseline in FVC were weak but increased over 52 weeks (r= –0.01 at week 4 and r=0.25 at week 52). Similar correlations were observed for FEV6 (r= –0.01 at week 4 and r=0.27 at week 52) (figure 3b).
FIGURE 4.
Changes from baseline in forced vital capacity (FVC) based on a) home spirometry and b) clinic spirometry. Boxes indicate median and interquartile range (IQR); “plus” symbols (+) indicate mean. Whiskers indicate 1.5 IQR. Outliers are shown as squares.
Correlations between home- and clinic-measured rates of change in FVC were weak but increased over 52 weeks (r=0.00 and r=0.26 for rates of decline in FVC over 4 and 52 weeks, respectively). Similar correlations were observed for rates of change in FEV6 (r= –0.05 and r=0.29 over 4 and 52 weeks, respectively) (figure 3c).
Home and clinic spirometry in subjects who had an acute exacerbation
One subject in the nintedanib group had an acute exacerbation during the double-blind period and seven subjects who initially received placebo had an acute exacerbation during the nintedanib open-label period. Home and clinic measurements of FVC before and after these acute exacerbations are presented in supplementary figure S5.
Discussion
In the INMARK trial conducted in subjects with IPF and preserved lung function, adherence to weekly home spirometry over 52 weeks was above 75% in every 4-week period, but decreased over time. Over 52 weeks, 31% of subjects adhered to the request to provide at least one measurement per week for all the weeks they were in the trial. A proportion of subjects provided more measurements than the minimum requested, with an average of three measurements per subject per week. These findings are consistent with previous studies in patients with ILDs that have demonstrated high adherence to daily or weekly home spirometry, but with high variability among individuals and a reduction in the number of measurements provided over time [6, 8, 14]. Previous work suggests that patients with IPF find home spirometers easy to use and not burdensome, and that patients like to see their FVC results to feel more in control of their disease [6, 15, 16]. A study of 30 subjects found that only four were unable to use the home spirometry device [6].
Within-subject variability in FVC measurements taken day-to-day or week-to-week has been observed in healthy individuals [17] as well as in subjects with IPF [4, 6]. The literature is inconsistent with respect to diurnal variations in FVC; several studies have found FVC to be generally higher in the morning than in the afternoon [18–20], but this has not been observed in all studies [21]. In the INMARK trial, subjects were asked to perform spirometry in the morning, but fewer than a third of subjects adhered to this request. The mean of FVC measurements taken in the morning was almost the same as the mean of measurements taken in the afternoon/evening, but, consistent with a previous study [20], variability appeared to be greater in measurements taken in the afternoon/evening than in the morning.
Consistent with previous studies [4, 6–8, 15, 16], we found that home and clinic measurements of FVC at individual visits were strongly correlated. However, there was only a weak correlation between home- and clinic-based measurements of changes in FVC. This appeared to be largely due to variability in changes in FVC measured using home spirometry, which was much greater than the variability observed using clinic spirometry. Errors in measurements taken at different time-points accumulate, such that measurement error has a greater impact on assessments of changes in FVC over time, which are based on several measurements, than on measurements taken at single time-points. While it may be hypothesised that more frequent home spirometry (i.e. more data points) might provide a more accurate estimate of lung function, in our study correlations between home and clinic measurements of FVC were weaker in subjects who provided more spirometry measurements per week, likely due to a greater number of outliers. This was observed despite the home spirometry device selecting the highest of three readings for every measurement. Improving the accuracy of home-based spirometry might overcome this problem. To date, no head-to-head comparisons of different spirometers have been undertaken to assess whether particular devices are easier to use correctly and associated with lower measurement error. The correlations between home- and clinic-measured FVC at baseline and at week 52 were the same, suggesting that there was no increase in the reliability of home spirometry during the trial. It has been proposed that the abbreviated FEV6 manoeuvre may be easier for patients to perform than measurement of FVC and so improve reproducibility among unsupervised subjects [22]. However, in our analyses the correlations between home and clinic measurements of FVC were almost the same as the correlations between home and clinic measurements of FEV6.
It has been postulated that more frequent measurement of FVC at home might enable earlier detection of an acute exacerbation. In a pilot study performed in 10 subjects, a decline in FVC based on daily home spirometry was observed 2 days before symptoms of a respiratory tract infection [15]. We were unable to perform a robust investigation into whether acute exacerbations could be detected earlier using more frequent home spirometry using our data given the small number of acute exacerbations reported in this population with very well preserved FVC at baseline and the low frequency of home spirometry measurements around the time of acute exacerbations.
Although not observed in the INMARK trial, technical issues with home spirometry devices and analytical issues arising from missing data have affected the analysis of home spirometry data from clinical studies in patients with ILDs [23], including trials of potential new therapies [10, 24]. More data are needed to inform strategies to ensure the quality of readings and reduce the variability of measurements obtained using home spirometry by better educating and motivating patients on the use of spirometry devices. It might be possible to reduce the amount of missing data and the variability of home spirometry measurements via local support from nurses or other healthcare professionals, or via closer or more regular examination of data so that any issues can be addressed promptly with the patient. A recent 24-week study in 90 patients with IPF that investigated the utility of a home monitoring programme integrating daily home spirometry, patient-reported outcomes, adverse event reporting, an information library and electronic consultations found home spirometry to be a reliable and accurate way of monitoring FVC [16]. Median adherence to daily home spirometry over 24 weeks was high (97%), and correlations between home- and hospital-based measurements of FVC were strong at all time-points. Unlike in the INMARK trial, in this study the correlation between the rates of change in home- and hospital-based measurements of FVC was moderately strong (r=0.58) [16].
Strengths of our analyses include the prospective multicentre design and the high frequency and volume of clinic and home spirometry measurements collected. Our findings also have limitations, including selection bias in the subjects who participated in the study, all of whom had preserved lung function at baseline, had shown a degree of adherence to home spirometry before entering the study and had chosen to enter a study that required home spirometry. We were unable to investigate whether comorbid asthma or chronic obstructive pulmonary disease had an impact on spirometry as so few patients in our study had these comorbidities. Our study did not collect data on subjects’ opinions (positive or negative) of home spirometry or on the reasons behind adherence/nonadherence to home spirometry.
In conclusion, in patients with IPF and preserved lung function, adherence to weekly home spirometry decreased over 52 weeks but remained high. Strong correlations were observed between FVC measurements obtained at home and in clinic at individual time-points, but correlations between changes in FVC measurements over time estimated using home and clinic spirometry were weak, mainly due to variability in the measurements obtained using home spirometry. At a group level, the rate of decline in FVC over 52 weeks was similar when measured using home or clinic spirometry. More data are needed on the utility of home spirometry as a means of measuring disease progression in patients with IPF in clinical trials and clinical practice.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01518-2020.SUPPLEMENT (737.8KB, pdf)
Shareable PDF
Acknowledgements
The authors thank the patients who participated in this trial. The authors did not receive payment for development of this manuscript. Writing assistance was provided by Julie Fleming and Wendy Morris of FleishmanHillard Fishburn (London, UK), which was contracted and funded by Boehringer Ingelheim. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Footnotes
This article has supplementary material available from erj.ersjournals.com
A video abstract describing the key data presented in this article is available at www.globalmedcomms.com/respiratory/noth/homespirometry
Data sharing: Data from the INMARK trial (ClinicalTrials.gov: NCT02788474) are available upon on request. A request can be submitted via https://trials.boehringer-ingelheim.com/trial_results/clinical_submission_documents.html
Conflict of interest: I. Noth reports personal fees for advisory board work from Boehringer Ingelheim and Genentech, personal fees for consultancy from ImmuneWorks, outside the submitted work.
Conflict of interest: V. Cottin reports personal fees for advisory board work and lectures, and nonfinancial support for meeting attendance from Actelion, grants, personal fees for consultancy and lectures, and nonfinancial support for meeting attendance from Boehringer Ingelheim, personal fees for advisory board and data monitoring committee work from Bayer/MSD and Galapagos, personal fees for lectures and advisory board work from Novartis, personal fees for consultancy and lectures, and nonfinancial support for meeting attendance from Roche, personal fees for lectures from Sanofi, personal fees for data monitoring and steering committee work from Promedior, personal fees for data monitoring work from Celgene and Galecto, outside the submitted work.
Conflict of interest: N. Chaudhuri reports grants, personal fees for advisory board work and educational support to attend conferences from Roche and Boehringer Ingelheim, outside the submitted work.
Conflict of interest: T.J. Corte reports grants and personal fees for travel, lectures and advisory board work from Boehringer Ingelheim, grants and personal fees for travel, lectures, steering committee work and advisory board work from Roche, grants from Gilead, Bayer and Intermune, personal fees for advisory board work from AstraZeneca and Ad Alta, grants and personal fees for steering committee and advisory board work from Bristol-Myers Squibb, personal fees for steering committee work from Promedior, during the conduct of the study.
Conflict of interest: K.A. Johannson reports personal fees for advisory board work, consultancy and lectures from Boehringer Ingelheim, personal fees for advisory board work and lectures from Hoffman La Roche Ltd, personal fees for advisory board work and consultancy from Theravance and Blade Therapeutics, grants from Chest Foundation, University of Calgary School of Medicine, Pulmonary Fibrosis Society of Calgary and UCB Biopharma SPRL, personal fees for consultancy from Three Lakes Foundation, outside the submitted work.
Conflict of interest: M. Wijsenbeek reports grants and personal fees from Boehringer Ingelheim and Hoffman La Roche, personal fees from Galapagos and Respivant, outside the submitted work; this article was based on discussions held at a meeting supported by Boehringer Ingelheim in June 2017.
Conflict of interest: S. Jouneau reports fees, funding or reimbursement for national and international conferences, boards, expert or opinion groups, research projects from Actelion, AIRB, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Chiesi, Galecto, Gilead, GlaxoSmithKline, LVL, Mundipharma, Novartis, Pfizer, Roche and Savara-Serendex, outside the submitted work.
Conflict of interest: A. Michael is a contractor to Boehringer Ingelheim.
Conflict of interest: M. Quaresma is an employee of Boehringer Ingelheim International GmbH.
Conflict of interest: K.B. Rohr is an employee of Boehringer Ingelheim International GmbH.
Conflict of interest: A-M. Russell reports grants and personal fees for meeting attendance from Boehringer Ingelheim, grants from Imperial Health Charity, Pulmonary Fibrosis Trust UK and Action for Pulmonary Fibrosis, personal fees for lectures from the Irish Lung Fibrosis Association and Hoffman La Roche, outside the submitted work.
Conflict of interest: S. Stowasser is an employee of Boehringer Ingelheim International GmbH.
Conflict of interest: T.M. Maher reports personal fees for advisory board work, consultancy or clinical trial work from Apellis, Boehringer Ingelheim, Roche, Bayer, Biogen Idec, Galapagos, Indalo, Galecto, Blade, Bristol-Myers Squibb, Novartis, Respivent and Trevi, grants and personal fees from UCB, grants and personal fees from GlaxoSmithKline, outside the submitted work.
Support statement: The INMARK trial was funded by Boehringer Ingelheim. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Raghu G, Remy-Jardin M, Myers JL, et al. . Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. doi: 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 2.Doubková M, Švancara J, Svoboda M, et al. . EMPIRE Registry, Czech part: impact of demographics, pulmonary function and HRCT on survival and clinical course in idiopathic pulmonary fibrosis. Clin Respir J 2018; 12: 1526–1535. doi: 10.1111/crj.12700 [DOI] [PubMed] [Google Scholar]
- 3.Jo HE, Glaspole I, Moodley Y, et al. . Disease progression in idiopathic pulmonary fibrosis with mild physiological impairment: analysis from the Australian IPF registry. BMC Pulm Med 2018; 18: 19. doi: 10.1186/s12890-018-0575-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell AM, Adamali H, Molyneaux PL, et al. . Daily home spirometry: an effective tool for detecting progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2016; 194: 989–997. doi: 10.1164/rccm.201511-2152OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maher TM. Home spirometry for idiopathic pulmonary fibrosis: ready for prime time? Eur Respir J 2017; 50: 1701403. doi: 10.1183/13993003.01403-2017 [DOI] [PubMed] [Google Scholar]
- 6.Johannson KA, Vittinghoff E, Morisset J, et al. . Home monitoring improves endpoint efficiency in idiopathic pulmonary fibrosis. Eur Respir J 2017; 50: 1602406. doi: 10.1183/13993003.02406-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcoux V, Wang M, Burgoyne SJ, et al. . Mobile health monitoring in patients with idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2019; 16: 1327–1329. doi: 10.1513/AnnalsATS.201904-335RL [DOI] [PubMed] [Google Scholar]
- 8.Swigris JJ, Nathan SD, Tighe RM, et al. . STARMAP: an observational study to assess disease-relevant outcomes using home monitoring devices in patients with idiopathic pulmonary fibrosis (IPF). Eur Respir J 2019; 54: Suppl. 63, PA1333. doi: 10.1183/13993003.congress-2019.PA1333 [DOI] [Google Scholar]
- 9.Wijsenbeek M, Bendstrup E, Valenzuela C, et al. . Design of a study assessing disease behaviour during the peri-diagnostic period in patients with interstitial lung disease: the STARLINER Study. Adv Ther 2019; 36: 232–243. doi: 10.1007/s12325-018-0845-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maher TM, Corte TJ, Fischer A, et al. . Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2020; 8: 147–157. doi: 10.1016/S2213-2600(19)30341-8 [DOI] [PubMed] [Google Scholar]
- 11.Maher TM, Stowasser S, Nishioka Y, et al. . Biomarkers of extracellular matrix turnover in patients with idiopathic pulmonary fibrosis given nintedanib (INMARK study): a randomised, placebo-controlled study. Lancet Respir Med 2019; 7: 771–779. doi: 10.1016/S2213-2600(19)30255-3 [DOI] [PubMed] [Google Scholar]
- 12.Quanjer PH, Tammeling GJ, Cotes JE, et al. . Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16: 5–40. doi: 10.1183/09041950.005s1693 [DOI] [PubMed] [Google Scholar]
- 13.Richeldi L, du Bois RM, Raghu G, et al. . Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. doi: 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 14.Wijsenbeek MS, Bendstrup E, Valenzuela C, et al. . Interim analysis of patients with ILD enrolled in the STARLINER study. Eur Respir J 2019; 54: Suppl. 63, PA1335. doi: 10.1183/13993003.congress-2019.PA1335 [DOI] [Google Scholar]
- 15.Moor CC, Wapenaar M, Miedema JR, et al. . A home monitoring program including real-time wireless home spirometry in idiopathic pulmonary fibrosis: a pilot study on experiences and barriers. Respir Res 2018; 19: 105. doi: 10.1186/s12931-018-0810-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moor CC, Mostard RLM, Grutters JC, et al. . Home monitoring in patients with idiopathic pulmonary fibrosis: a randomized controlled trial. Am J Respir Crit Care Med 2020; 202: 393–401. doi: 10.1164/rccm.202002-0328OC [DOI] [PubMed] [Google Scholar]
- 17.Rozas CJ, Goldman AL. Daily spirometric variability: normal subjects and subjects with chronic bronchitis with and without airflow obstruction. Arch Intern Med 1982; 142: 1287–1291. doi: 10.1001/archinte.1982.00340200045012 [DOI] [PubMed] [Google Scholar]
- 18.Borsboom GJ, van Pelt W, van Houwelingen HC, et al. . Diurnal variation in lung function in subgroups from two Dutch populations: consequences for longitudinal analysis. Am J Respir Crit Care Med 1999; 159: 1163–1171. doi: 10.1164/ajrccm.159.4.9703106 [DOI] [PubMed] [Google Scholar]
- 19.Fregonezi G, Resqueti VR, Cury JL, et al. . Diurnal variations in the parameters of pulmonary function and respiratory muscle strength in patients with COPD. J Bras Pneumol 2012; 38: 257–263. doi: 10.1590/S1806-37132012000200016 [DOI] [PubMed] [Google Scholar]
- 20.Moor CC, van den Berg CAL, Visser LS, et al. . Diurnal variation in forced vital capacity in patients with fibrotic interstitial lung disease using home spirometry. ERJ Open Res 2020; 6: 00054-2020. doi: 10.1183/23120541.00054-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medarov BI, Pavlov VA, Rossoff L. Diurnal variations in human pulmonary function. Int J Clin Exp Med 2008; 1: 267–273. [PMC free article] [PubMed] [Google Scholar]
- 22.Vandevoorde J, Verbanck S, Schuermans D, et al. . FEV1/FEV6 and FEV6 as an alternative for FEV1/FVC and FVC in the spirometric detection of airway obstruction and restriction. Chest 2005; 127: 1560–1564. doi: 10.1378/chest.127.5.1560 [DOI] [PubMed] [Google Scholar]
- 23.Maher TM, Swigris J, Moor C, et al. . Home spirometry as an endpoint in interstitial lung diseases clinical trials – lessons learned. 2020. https://euipfsummit.org/wp-content/uploads/2020/07/Home-Spirometry-As-An-Endpoint-In-Interstitial-Lung-Disease-ILD-Clinical-Trials_poster.pdf Date last accessed: December 20, 2020.
- 24.Maher TM, Corte TJ, Kreuter M, et al. . Home spirometry as a primary endpoint in clinical trials: sensitivity analyses of a randomized controlled trial of pirfenidone in patients with unclassifiable interstitial lung disease (UILD). Am J Respir Crit Care Med 2020; 201: Suppl., A2575. doi: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A2575 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01518-2020.SUPPLEMENT (737.8KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01518-2020.Shareable (434.4KB, pdf)