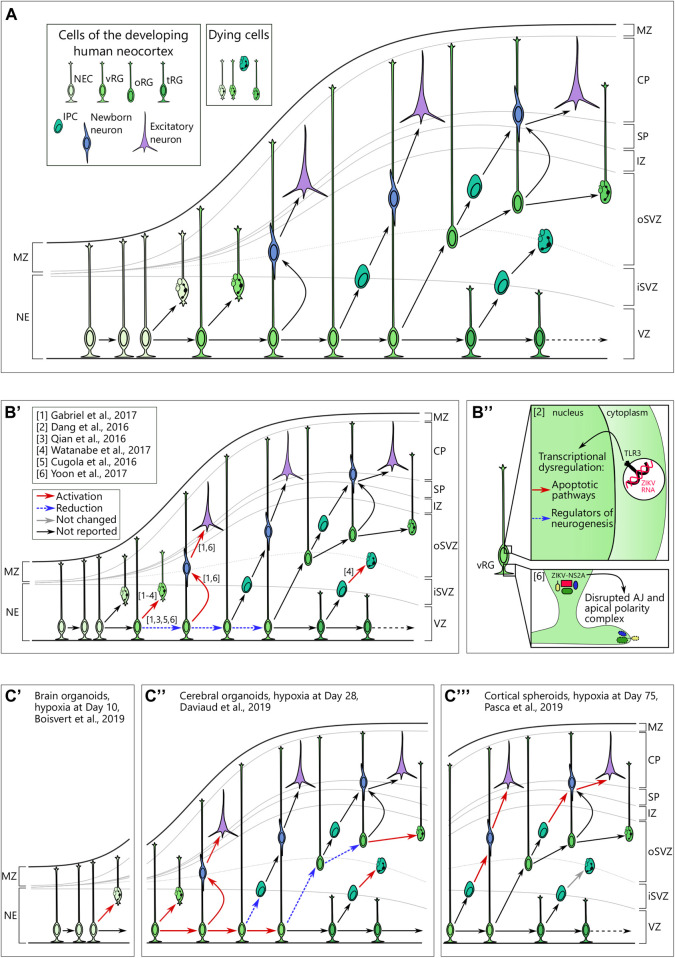
FIGURE 2.
(A) Cellular view of the normal human neocortical development. Human neocortical development starts from neuroepithelial cells (NEC), which further transform to ventricular radial glia (vRG). Both NEC and vRG have nuclei next to the ventricle (neuroeplithelium (NE) and ventricular zone (VZ), respectively) and a basal process spanning through the cortical wall and marginal zone (MZ) to reach the pial surface. vRG can either divide symmetrically to generate two vRGs or differentiate to produce excitatory neurons or basal neural progenitor cells. Basal progenitor cells reside in the inner (iSVZ) and outer (oSVZ) subventricular zones and include intermediate progenitor cells (IPC) and outer radial glia (oRG). Both types of basal neural progenitor cells can self-amplify or differentiate into excitatory neurons. Later in development, vRG lose connection to the pial surface and transform into truncated radial glia (tRG) while still generating basal progenitors and excitatory neurons. Newborn neurons of different origins migrate along the radial fibers through the intermediate zone (IZ) and subplate (SP) and take their place in the cortical plate (CP). A low level of cell death in neural cells is normal over neurogenesis. (B) Brain organoids infected with Zika virus reveal cellular mechanisms of microcephaly. B’. Multiple studies suggest preferential infection of neural progenitor cells over neurons. Infection in vRG leads to the apoptotic cell death, attenuation of proliferation as well as shift to direct neurogenesis. (B’’) upper panel. Zika virus (ZIKV) activates Toll-like receptor 3 in vRGs that leads to transcriptional deregulation with activation of apoptotic pathways and inhibition of regulators of neurogenesis. Adapted from Dang et al. (2016). (B’’) lower panel. NS2A, a protein of ZIKV envelop, binds the components of adherens junctions (AJ) in the cytoplasm and prevents them from forming functional AJ and apical polarity complex. Reprinted from Yoon et al. 2017 with permission from Elsevier. Copyright (2017). (C) Hypoxic exposure in brain organoids at different time points results in distinct defects on the cellular level. (C’) Hypoxic exposure (1% O2, 72 h) at day 10 of organoid differentiation results in the increased cell death, presumably, of NECs. (C’’) When hypoxia (3% O2, 24 h) is applied to brain organoids at day 28, it results in the immediate cell death across the cortical wall followed by proliferation in vRG. Subsequently, vRG tend to differentiate into neurons at the expense of generation of both IPCs and oRG, which results in the decreased number of these cells 14 days after hypoxic exposure. (C’’’) Cortical spheroids that are exposed to hypoxia (1% O2, 48 h) at day 75 of differentiation show decreased numbers of IPCs resulting from premature differentiation but not from cell death in these cells.