FIGURE 1.
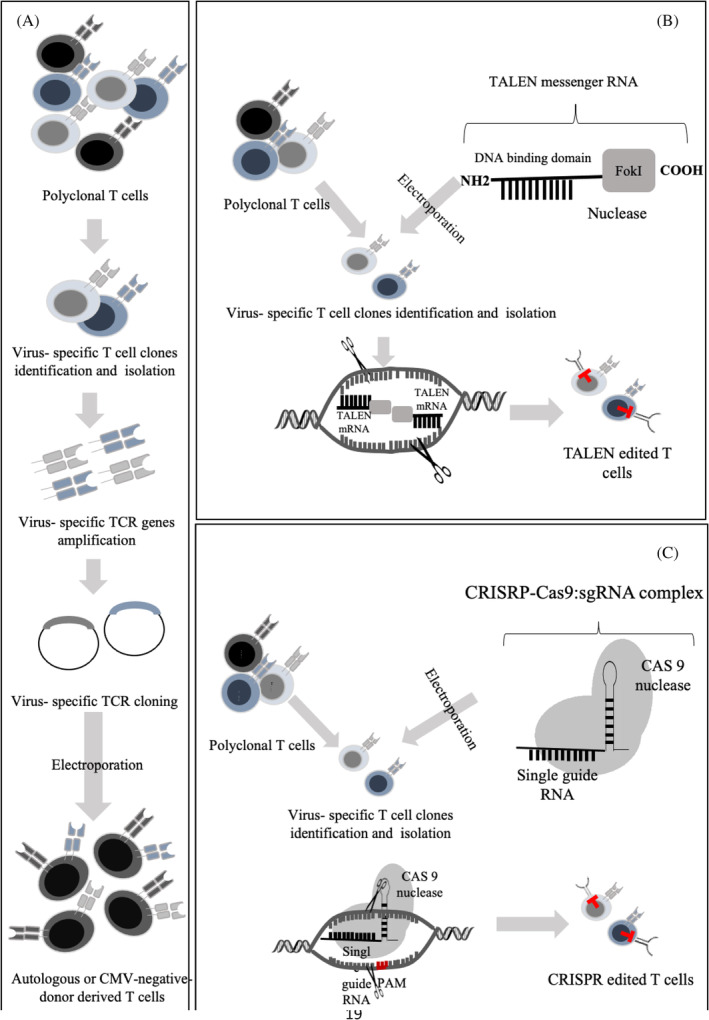
Virus‐specific engineered T cells. (A) Generation of TCR‐edited T cells: virus‐specific T cell isolation from polyclonal T cell population is performed via different methods, including co‐culturing with different HLA I‐ and II‐ restricted epitopes of virus specific Ag, cytokine capture system, and/or peptide‐MHC multimer. RNA from T cell clones is extracted, cDNA is synthesized, and TCR α and β chains are amplified via PCR and then analyzed. TCR α and β chains are separately inserted into viral vectors, amplified, and verified by DNA sequencing. Patient‐ or donor‐derived PBMCs are activated and transduced by viral vectors containing virus‐specific TCR chain genes to generate virus‐specific T cells. (B) Generation of drug‐resistant T cells (TALEN‐edited T cells): virus‐specific T cells are isolated and expanded from polyclonal T cell population. TALEN, as restriction enzyme, is engineered to cut specific DNA sequences of drug receptor (eg, glucocorticoid receptor). TALEN messenger RNA is transferred into expanded virus‐specific T cells by electroporation. Drug receptor‐targeting TALEN causes site‐specific double‐stranded DNA breaks and triggers natural DNA repair (nonhomologous end joining) that induces deletions of drug receptor gene in T cells. Finally, drug resistance is experimented in vitro and in vitro and functional capacity of TALEN‐modified T cells is confirmed. (C) CRISPR‐Cas9‐edited T cells: CRISPR‐Cas9, as an RNA‐programmable DNA targeting tool, could target specific genes and knock out drug receptors. CRISPR‐Cas9 induces site‐specific double‐stranded breaks in the target DNA in the presence of a short protospacer adjacent motif (PAM) flanking the target site. After the target site recognition, and following complementary base pairing between the synthetic guide RNA and target DNA, R‐loop is formed and cut into DNA strands. Then, Cas9 interacts with DNA that leads to conformational changes. 50 Virus‐specific T cells are isolated from polyclonal T cell population and expanded. Specific drug receptor knocked‐out by CRISPR/Cas9 can be transferred into isolated virus‐specific T cells by electroporation. Knock‐out efficiency is evaluated via PCR amplification and DNA sequencing of PCR products
