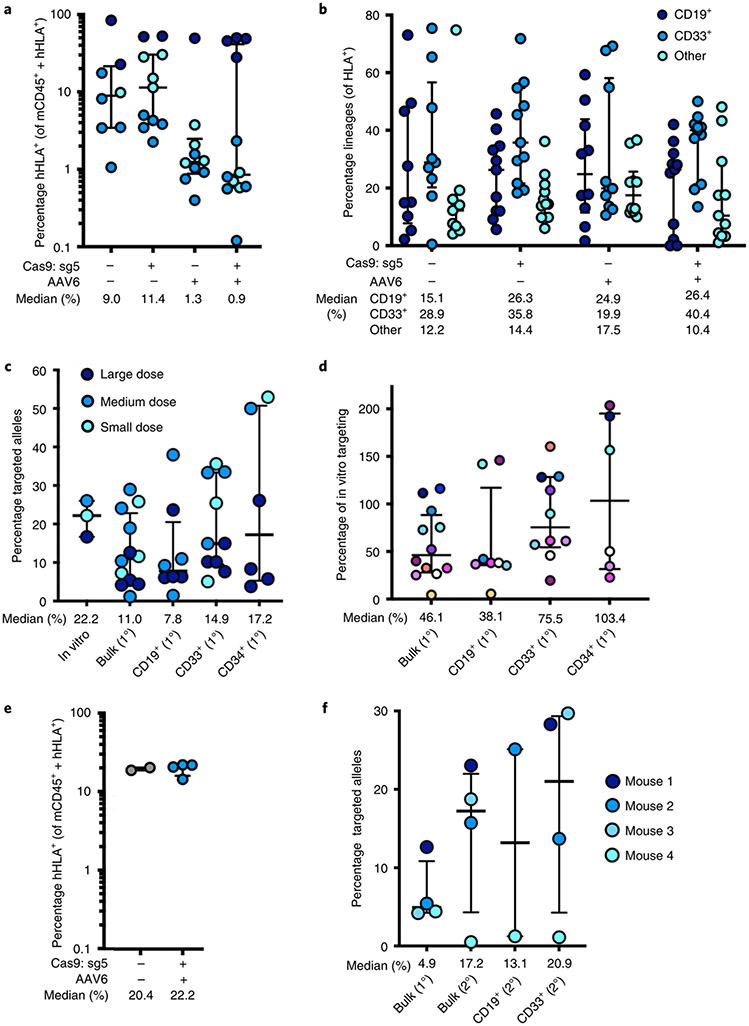
Fig. 4 ∣. Engraftment of α-globin-targeted human HSPCs into NSG mice.
a, Sixteen weeks after transplantation of edited human CD34+ HSPCs into mice, bone marrow was harvested and engraftment rates were determined. Depicted is the percentage of mTerr119− cells (non-RBCs) that were hHLA+ among the total number of mCd45+ or hHLA+ cells. Bars represent median ± interquartile range. Values represent biologically independent transplantations: n = 8 for mock, n = 11 for RNP only, n = 10 for AAV only and n = 12 for RNP + AAV. b, Among engrafted human cells, distribution among CD19+ (B-cell), CD33+ (myeloid) and other (that is, HSPC/RBC/T/NK/Pre-B) lineages. Bars represent median ± interquartile range. Values represent biologically independent transplantations: n = 9 for mock, n = 11 for RNP only and RNP + AAV and n = 10 for AAV only. c, Targeted allele frequency at HBA1 as determined by ddPCR among in vitro (pretransplantation) HSPCs and bulk engrafted HSPCs, as well as among lineages. Bars represent median ± interquartile range. n = 3 for in vitro HSPCs, n = 12 for bulk engrafted HSPCs, n = 8 for CD19+ HSPCs, n = 10 for CD33+ HSPCs and n = 6 for CD34+ HSPCs. d, Targeted allele frequency at HBA1 among engrafted human cells compared to targeting rate pretransplantation in an in vitro human HSPC population. Individual mice are represented by different colors. Bars represent median ± interquartile range. n = 12 for bulk engrafted HSPCs, n = 8 for CD19+ HSPCs, n = 10 for CD33+ HSPCs and n = 6 for CD34+ HSPCs. e, 16 weeks post secondary transplantation, bone marrow was harvested and engraftment rates were determined as above. Bars represent median ± interquartile range. n = 2 for mock and n = 4 for edited treatment groups. f, Targeted allele frequency at HBA1 determined by ddPCR among engrafted human cells in bulk sample and lineages in secondary transplantations. Bars represent median ± interquartile range. n = 4 for each treatment group, with the exception of CD19+ HSPCs (n = 2). Large, medium and small doses correspond to 1,300,000, 750,000 and 250,000 transplanted cells, respectively. 1° and 2° indicate analysis of engraftment human cells harvested from primary or secondary mouse transplantations, respectively.