Figure 2.
Pneumococcal PspA interacts with human GAPDH
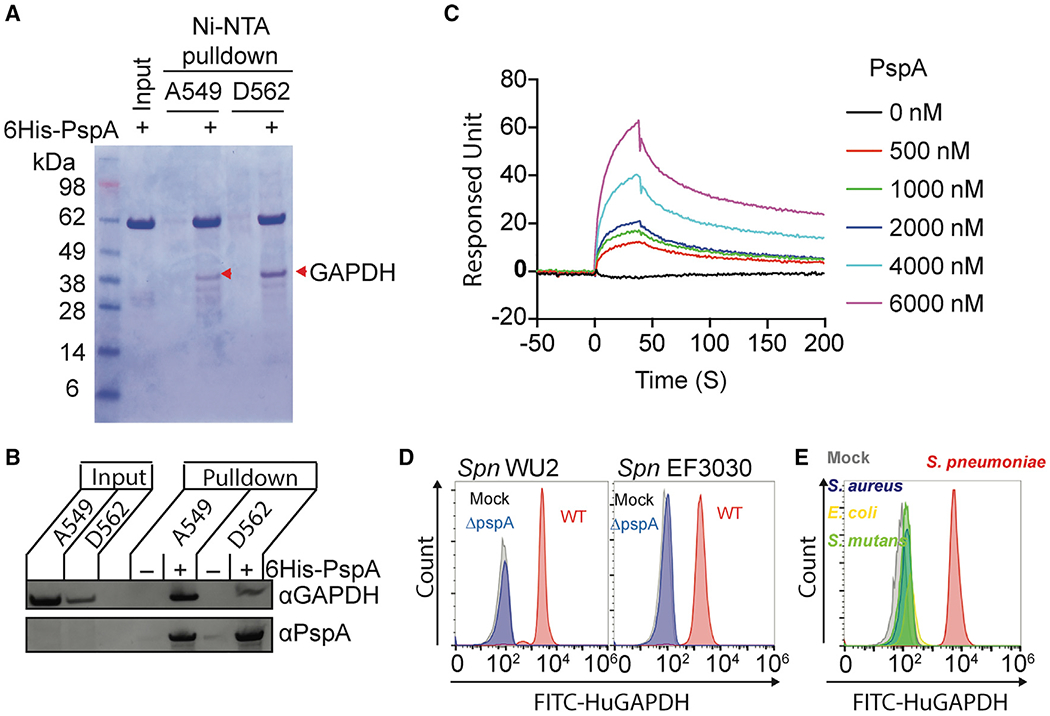
(A) Recombinant His-tagged PspA protein composed of the αHD and PRD was used in pulldown experiments with A549 and D562 cell lysates. The PspA bound proteins were separated by SDS-PAGE and visualized by Coomassie blue dye. Interacted proteins were identified by liquid chromatography tandem mass spectrometry (Table S1). Red arrowheads indicate GAPDH.
(B) Interaction of PspA with host GAPDH was analyzed by immunoblot after A549 and D562 cell lysates were pulled down using PspA as bait.
(C) Interactions of PspA with huGAPDH were analyzed by surface plasmon resonance spectroscopy under different PspA concentrations. (D) Spn WU2, Spn EF3030, or its pspA isogenic mutants, and (E) S. aureus, E. coli, and S.mutans were incubated with FITC-conjugated huGAPDH. The huGAPDH binding to bacterium was measured by flow cytometry. Mock represents Spn bacteria only.
Data are representative of (A and B) two or (C–D) three independent experiments.
