Figure 3.
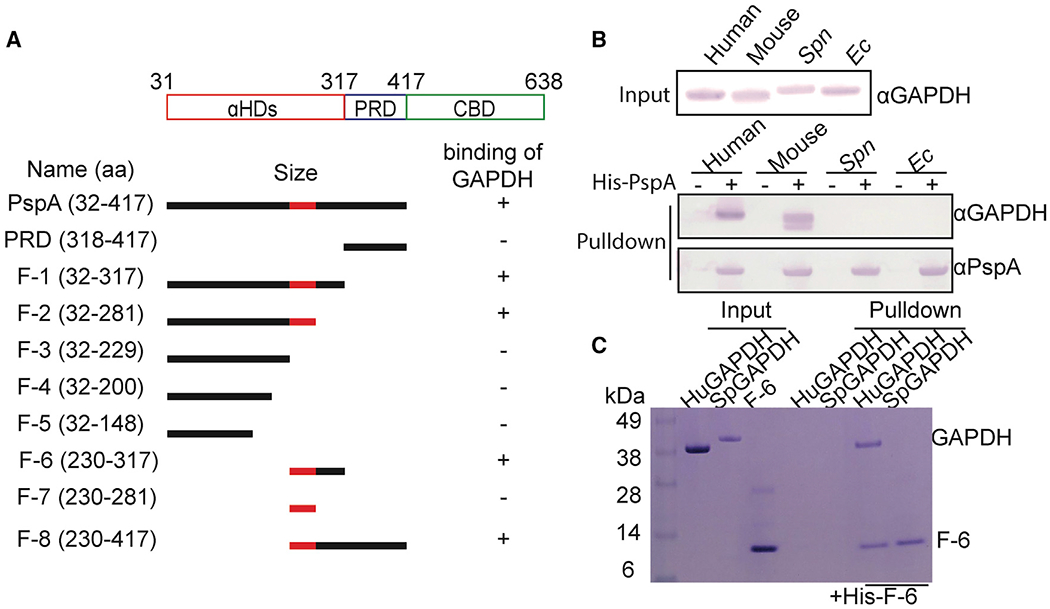
α-Helical domain (aa 230–281) of PspA is required for host GAPDH selectively
(A) Diagram of PspA and the corresponding rPspA fragments (F-) used in pull-down experiments. These truncated fragments were used to test PspA binding to GAPDH (see Figure S3). The region colored red within the αHD of PspA was identified as the subdomain that mediates GAPDH binding.
(B) Cell lysates from different sources were used in pull-down experiments using rPspA as bait. Versions of GAPDH that interacted with PspA were detected by immunoblot.
(C) F-6 was pulled down with purified human GAPDH and visualized by SDS-PAGE separation and staining with Coomassie blue dye. Results shown are representative with experiments performed three times with identical results.
