Figure 1.
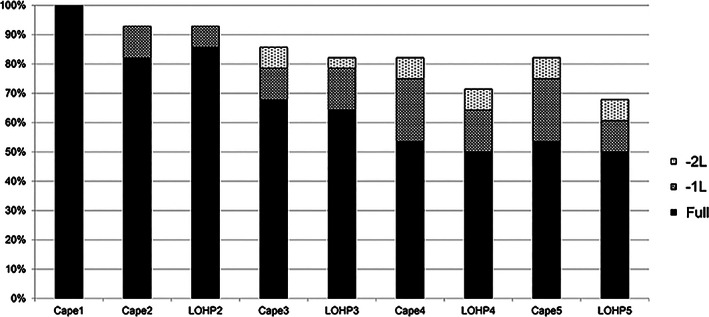
Dose modification and treatment continuation of adjuvant capecitabine plus oxaliplatin in combination (n = 28). Dose administered in each cycle is represented by individual bars. In cycle 1, capecitabine alone is administered (Cape1). In cycle 2, the dose of capecitabine is denoted by Cape2 and the dose of oxaliplatin as LOHP2. The y‐axis shows the percentage of patients receiving the indicated dose (full, –1 dose level, or –2 dose level). Full dose capecitabine was administered at 1,000 mg/m2 twice daily (total 2,000 mg/m2 per day); doses could be reduced to doses 0.8 times starting dose (–1 level) and doses 0.6 times starting dose (–2 level).
Abbreviations: –1L, –1 level; –2L, –2 level; Cape1, capecitabine dose in each case of the first cycle; LOHP2, oxaliplatin dose in each case of the second cycle
