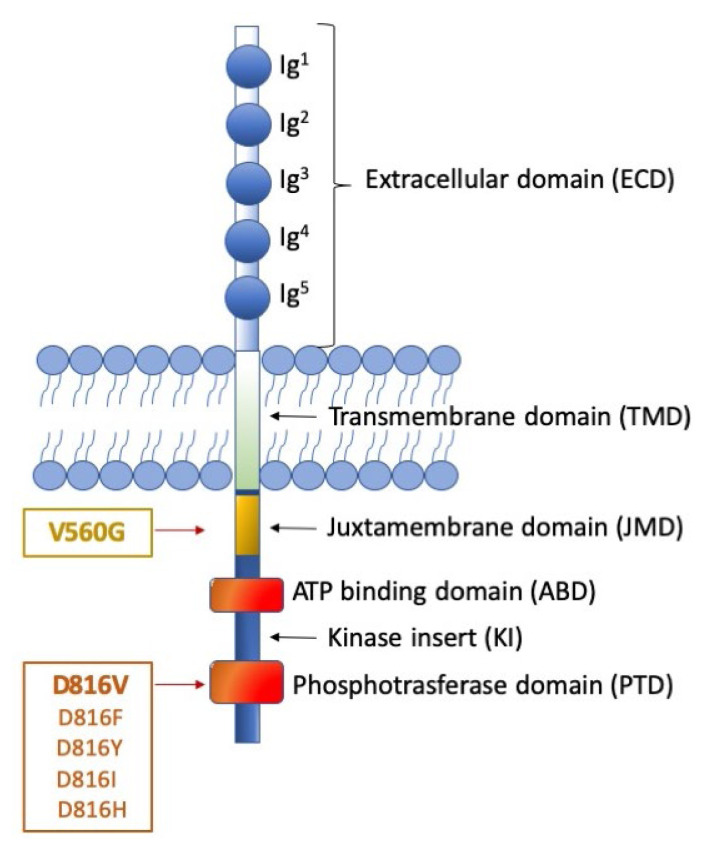
Figure 1. Representation of KIT receptor structure and position of the major mutations.
The figure shows the receptor under its monomeric form. The extracellular domain (ECD) (light blue) is characterized by 5 Immunoglobulin (Ig)-like domains that contain a ligand binding site for stem cell factor (SCF) and a dimerization site. The cytoplasmic region contains a transmembrane domain (light green) made by a single helix. The intracellular portion (dark blue) contains an auto-inhibitory juxta-membrane domain (yellow) and a kinase domain (orange) which is split into two parts: an ATP-binding domain and a phosphotransferase domain, linked by a kinase insert. On the left, two distinct categories of KIT activating mutations are reported: the ‘regulatory type’ mutations (with the V560G being the most frequent), that are located in the juxta-membrane domain; and the ‘enzymatic pocket’ mutations (best exemplified by the D816 mutations), that fall in the phosphotransferase domain. Though much rarer, the first category of mutations can be addressed by a wider spectrum of inhibitors, including imatinib. The second category of mutations, in contrast, impacts on kinase conformation, thus can be addressed, at present, only by midostaurin (approved) avapritinib and ripretinib (investigational).