Abstract
Background:
Teratogenic potential has been linked to various industrial compounds. Methoxyacetic acid (MAA) is a primary metabolite of the widely used organic solvent and plasticizer, methoxyethanol and dimethoxyethyl phthalate, respectively. Studies using model animals have shown that MAA acts as the proximate teratogen that causes various malformations in developing embryos. Nonetheless, the molecular mechanisms by which MAA exerts its teratogenic effects are not fully understood.
Methods:
Gastruloids of mouse P19C5 pluripotent stem cells, which recapitulate axial elongation morphogenesis of gastrulation-stage embryos, were explored as an in vitro model to investigate the teratogenic action of MAA. Morphometric parameters of gastruloids were measured to evaluate the morphogenetic effect, and transcript levels of various developmental regulator genes were examined to assess the impact on gene expression patterns. The effects of MAA on the level of retinoic acid (RA) signaling and histone deacetylase activity were also measured.
Results:
MAA reduced axial elongation of gastruloids at concentrations comparable to the teratogenic plasma level (5 mM) in vivo. MAA at 4 mM significantly altered the expression profiles of developmental regulator genes. In particular, it upregulated the RA signaling target genes. The concomitant suppression of RA signaling using a pharmacological agent alleviated the morphogenetic effect of MAA. MAA at 4 mM also significantly reduced the activity of purified histone deacetylase protein.
Conclusions:
MAA impaired axial elongation morphogenesis in a RA signaling-dependent manner in mouse gastruloids, possibly through the inhibition of histone deacetylase.
Keywords: gastruloids, histone deacetylase, methoxyacetic acid, morphogenesis, retinoic acid
1 |. INTRODUCTION
Teratogenic potential has been linked to various industrial and pharmaceutical compounds, which women of childbearing age may be exposed to (Schardein & Macina, 2006). Understanding how these chemicals affect embryo development at the molecular level may help identify other potentially teratogenic compounds and lead to strategies to alleviate their adverse effects. However, in vivo studies using human and model animals are often too complicated to elucidate the molecular mechanisms of teratogenic compounds. This is largely because the properties and concentrations of compounds are dynamically altered in the mother through absorption, distribution, metabolism, and excretion. By contrast, in vitro models of embryogenesis, such as those created from pluripotent stem cells, are devoid of such maternal influences, and are more amenable to various experimental interrogations for mechanistic analyses. Thus, in vitro approaches can augment in vivo studies by providing valuable insights into the molecular actions of teratogenic compounds.
In vitro gastrulation models called, gastruloids, have been generated from pluripotent stem cells, which recapitulate axial elongation morphogenesis of gastrulation-stage embryos (Beccari et al., 2018; Marikawa, Tamashiro, Fujita, & Alarcón, 2009; van den Brink et al., 2014). In the case of gastruloids made from mouse P19C5 stem cells, three-dimensional cell aggregates grow in size and transform from a spherical to elongated shape with a distinct anterior–posterior axis within 4 days of hanging drop culture (Lau & Marikawa, 2014). Growth and axial morphogenesis of P19C5 gastruloids are significantly impaired by various teratogenic chemicals at the physiologically relevant concentrations (Li & Marikawa, 2016; Warkus & Marikawa, 2017; Warkus, Yuen, Lau, & Marikawa, 2016). Gene expression patterns in gastruloids are also altered by the inhibition of the morphogenetic signals, such as WNT, NODAL, NOTCH, and retinoic acid (RA), in a manner consistent with their functions in normal embryonic patterning (Li & Marikawa, 2015). Accordingly, P19C5 gastruloids have been explored as an in vitro tool to identify molecular pathways linked to the teratogenic effects of various chemicals, such as valproic acid (Li & Marikawa, 2016), fluoxetine (Warkus & Marikawa, 2018), and resveratrol (Kim & Marikawa, 2018).
The purpose of the present study is to investigate teratogenic mechanisms of methoxyacetic acid (MAA), using the mouse gastruloid. MAA (linear formula: CH3OCH2COOH) is a primary metabolite of organic solvent methoxyethanol (ME) and of plasticizer dimethoxyethyl phthalate (DMEP). Studies using model animals, ranging from rodents to nonhuman primates, have unequivocally demonstrated that MAA, but not ME or DMEP, is the proximate teratogen, which causes various malformations, such as defects in the neural tube, heart, kidneys, limbs, and tail (Brown, Holt, & Webb, 1984; Clarke, Duignan, & Welsch, 1992; Ritter, Scott Jr., Randall, & Ritter, 1985; Scott, Fradkin, Wittfoht, & Nau, 1989; Sleet, Greene, & Welsch, 1988; Terry, Elswick, Stedman, & Welsch, 1994; Yonemoto, Brown, & Webb, 1984). MAA is one of the short-chain fatty acids that activate mitogen-activated protein kinases and inhibit histone deacetylases (HDAC) to enhance estrogen receptor activity (Jansen et al., 2004). Inhibition of HDAC, in particular, is considered to be involved in the teratogenic effects of MAA (Dayan & Hales, 2014). Nonetheless, how such molecular effects of MAA are linked to the pathogenesis of specific birth defects is not fully understood. A better understanding of the molecular pathways that are affected by MAA is crucial to evaluate the reproductive risk in human, which may result from exposures to the widely distributed ME and DMEP.
Here, we showed that MAA significantly altered morphogenesis and gene expression profile in mouse gastruloids at the teratogenic concentrations. Our studies suggest that enhanced transcription of RA-signaling target genes through inhibition of HDAC is responsible for the adverse effect of MAA to impair axial elongation morphogenesis.
2 |. MATERIALS AND METHODS
2.1 |. Chemicals
All chemical compounds used in the present study were commercially obtained, namely from Sigma-Aldrich (St. Louis, MO), Santa Cruz Biotechnology (Dallas, TX), and Calbiochem (San Diego, CA), and their details are described in Table 1.
TABLE 1.
Chemicals used in the present study
| Chemical name | CASRN | Vendor (catalog number) | Stock concentration (solvent) |
|---|---|---|---|
| Methoxyacetic acid | 625-45-6 | Sigma-Aldrich (194557) | 1 M (water) |
| Methoxyethanol | 109-86-4 | Sigma-Aldrich (284467) | 12.7 M (undiluted) |
| Retinoic acid | 302-79-4 | Sigma-Aldrich (R2625) | 100 μM (DMSO) |
| Trichostatin A | 58880-19-6 | Calbiochem (647925) | 100 μM (DMSO) |
| Valproic acid | 1069-66-5 | Sigma-Aldrich (P4543) | 100 mM (water) |
| BMS493 | 215030-90-3 | Santa Cruz (sc-361124) | 10 mM (DMSO) |
Abbreviations: CASRN, chemical abstracts service registry number; DMSO, dimethyl sulfoxide.
2.2 |. Cell culture
P19C5 mouse embryonal carcinoma cells (Lau & Marikawa, 2014) were propagated in culture medium, consisting of 90% minimum essential medium alpha with nucleosides and GlutaMAX Supplement (LifeTechnologies, Carlsbad, CA), 2.5% fetal bovine serum, 7.5% newborn calf serum, 50 units/ml penicillin, and 50 μg/ml streptomycin. Gastruloids were generated as three-dimensional cell aggregates in hanging drops (Figure 1) of culture medium, containing 1% dimethyl sulfoxide (DMSO) with or without a test chemical, according to the method described previously (Lau & Marikawa, 2014). Monolayered cell culture was prepared by seeding 10,000 cells per well in 24-well plates with 500 μl of culture medium the day before chemical treatment.
FIGURE 1.
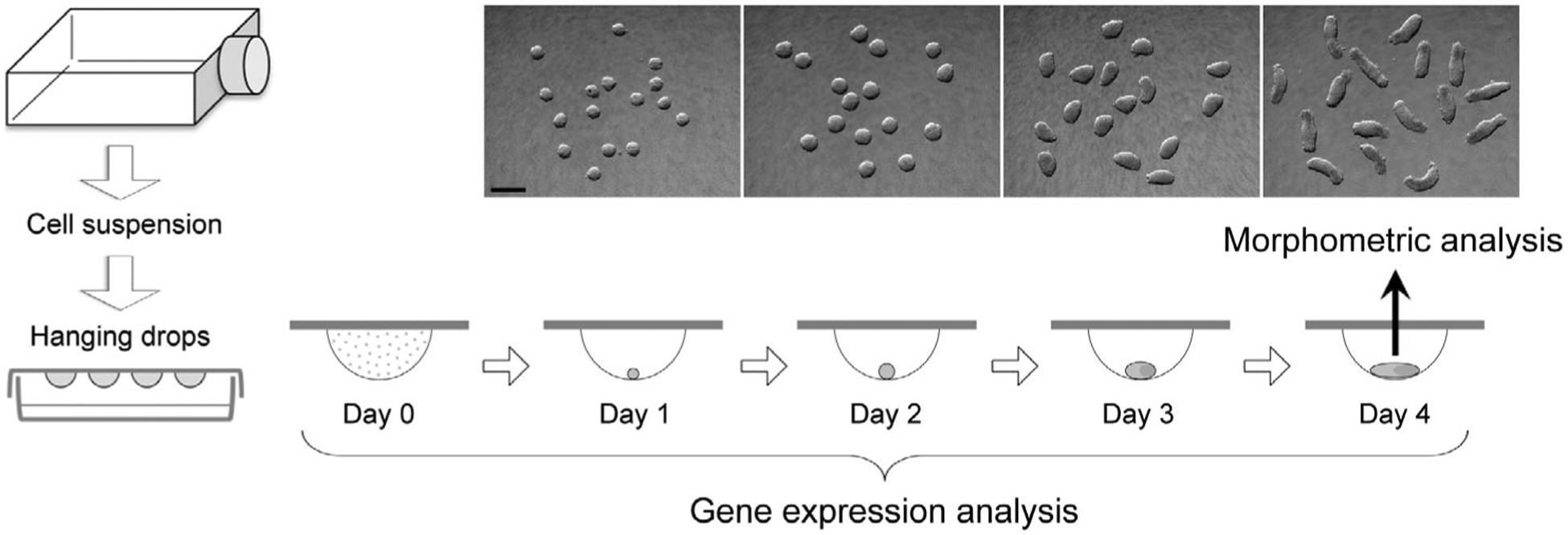
The experimental scheme to evaluate the morphogenetic and molecular effects of chemical exposures using gastruloids made from mouse P19C5 pluripotent stem cells. Representative images of gastruloids for each day of culture are shown. Scale bar = 500 μm
2.3 |. Morphometric analyses and criteria for adverse effects
Gastruloids were removed from hanging drops at Day 4 of culture (Figure 1) and placed together in a dish filled with phosphate-buffered saline for photography. Image files in JPEG format were opened in the ImageJ program (http://rsb.infonihgov/ij), and morphological parameters (area and aspect ratio) of each gastruloid were measured by manually tracing the circumference using the polygon selection tool. Aspect ratio is the ratio of the major-to-minor axis of an ellipse that most tightly fits the circumference of a gastruloid, and it is reflective of the extent of axial elongation (Li & Marikawa, 2015). Morphological assessment of chemical-treated gastruloids was conducted in three biological replicates using different collections of cell suspensions. For each replicate, 16 gastruloids were generated per chemical treatment in parallel with 16 control gastruloids (i.e., 1% DMSO only). Because the size and extent of elongation (reflected by area and aspect ratio, respectively) of control gastruloids were different among experimental replicates, normalization was performed relative to the average values of control gastruloids to yield relative area and relative aspect ratio, expressed as a percentage (i.e., control = 100%). Data of relative area and relative aspect ratio from all replicates were compiled, and their averages are shown with error bars of 95% confidence interval. In the present study, a chemical treatment was defined as having an adverse morphogenetic effect when it reduced the average relative area by >20% or the average aspect ratio by >40% compared with the control, according to the rationale described previously (Warkus & Marikawa, 2017).
2.4 |. Quantitative reverse transcription-polymerase chain reaction
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed to determine the relative expression levels of developmental regulator genes (Table 2). Total RNA was extracted from cell suspension before aggregation (Day 0) and gastruloids (control and MAA-treated) at Days 1, 2, 3, and 4 (Figure 1) using TRI reagent (LifeTechnologies) and Direct-zol RNA MiniPrep Kit (Zymo Research, Irvine, CA), and processed for cDNA synthesis using M-MLV Reverse Transcriptase (Promega, Madison, WI) and oligo-dT (18) primer. Quantitative PCR was performed using the CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA) with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) as follows: initial denaturation at 94 °C for 5 min, followed by up to 45 cycles of 94 °C for 15 s, 60 °C for 20 s, and 72 °C for 40 s. Data files were opened in CFX Manager software (Bio-Rad) and Ct values were transferred to the Excel program for further analyses. Actb, which encodes β-Actin, was used as a housekeeping gene to normalize the expression levels of other genes. Actb has been effectively used as a housekeeping gene in previous studies to evaluate gene expression levels in P19C5 gastruloids under various experimental conditions (Kim & Marikawa, 2018; Lau & Marikawa, 2014; Li & Marikawa, 2015, 2016; Warkus & Marikawa, 2018; Yuan & Marikawa, 2017). Additionally, based on the previous microarray analysis data (Kim & Marikawa, 2018), the transcript level of Actb is mostly stable from Days 0 to 4 of gastruloid culture, which is comparable or superior to other commonly used housekeeping genes, such as Gapdh, Hmbs, Hprt, Tbp, and Ywhaz (Figure S1). Gene expression analyses were conducted using three independent sets of samples as biological replicates using different collections of cell suspensions. Each set consisted of 9 samples: Day 0, control gastruloids at Days 1 to 4, and MAA-treated gastruloids at Days 1 to 4, all of which were originated from the same cell suspension. Relative expression levels were calculated for each set of experiment, as previously described (Warkus & Marikawa, 2018), and the averages of the three replicates are shown with error bars of standard deviations.
TABLE 2.
Developmental regulator genes examined in the present study
| Gene name | Characteristicsa | Primer sequences (5′ → 3′) | References |
|---|---|---|---|
| Actb |
|
F: GAGAGGGAAATCGTGCGTGACATC R: CAGCTCAGTAACAGTCCGCCTAGA |
|
| Aldh1a2 |
|
F: CTTGCCTCACAACAAGTGAGCTTC R: TCACCCAGGTTAGAGACTGGCTTC |
Haselbeck et al., 1999 |
| Brachyury |
|
F: CCTCGGATTCACATCGTGAGAGTT R: AGTAGGTGGGCGGGCGTTATGACT |
Herrmann, 1991 |
| Cdx1 |
|
F: TCAGGACTGGACATGAGGTAGAGG R: TGGGAAGGTGGGCATGAGCAGGTA |
Meyer & Gruss, 1993 |
| Cyp26a1 |
|
F: CGGAGCTGTGTAGGCAAAGAGTTT R: CCTGGAAGTGGGTAAATCTTGCAG |
Sakai et al., 2001 |
| Hoxa1 |
|
F: CCCTTTCCTTCCACACTGTCTTGT R: AAGACCCGTAAACTCTGCTCTGGA |
Wellik, 2009 |
| Lfng |
|
F: AAGCAGGGAGTGGTTTTATGAAGG R: GGGATAGGAATGTATGAATGGGGA |
Shifley et al., 2008 |
| Meox1 |
|
F: AAAAATCAGACTTCCCAGCGACAG R: TTCACACGTTTCCACTTCATCCTC |
Mankoo et al., 2003 |
| Mixl1 |
|
F: CGACAGACCATGTACCCAGACATC R: TGAGGCTTCAAACACCTAGCTTCA |
Hart et al., 2002 |
| Msgn1 |
|
F: CCAGAAAGGCAGCAAAGTCAAGAT R: TCTGTGAGTTCCCCGATGTACTTG |
Yoon & Wold, 2000 |
| Nanog |
|
F: GCTTTGGAGACAGTGAGGTGCATA R: GCTACCCTCAAACTCCTGGTCCTT |
Mitsui et al., 2003 |
| Notch1 |
|
F: GTCTGCAGGCTCCAGTGTTCTGTA R: TCAGTTGGATTTGGATGATGCTGT |
Huppert et al., 2000 |
| Snai1 |
|
F: CCGTCCAGCTGTAACCATGCCTCA R: TGGGAGACACATTGGCCAGGCTGA |
Carver et al., 2001 |
| Pou5f1 |
|
F: AGGCAGGAGCACGAGTGGAAAGCA R: GGAGGGCTTCGGGCACTTCAGAAA |
Nichols et al., 1998 |
| Tbx6 |
|
F: GGCCTCTCTTCCACCCTTTAGTTC R: CACTAGTAACAAGGCCCCCAGGAG |
Chapman et al., 1996 |
| Wnt3 |
|
F: CAGATGCCCGCTCAGCTATGAACA R: AGCAGCACCAGTGGAAGACGCAAT |
Liu et al., 1999 |
Characteristics are described based on: a. Molecular function, b. Major expression domains around the gastrulation stage (mouse embryonic stages from E5.5 to E8.5), and c. Functional significance in early embryo development.
2.5 |. Histone deacetylase activity assay
Histone deacetylase (HDAC) activity was measured using the HDAC-Glo I/II Assay System (Promega), which utilizes an acetylated, cell-permeable, luminogenic peptide as an HDAC substrate. Human recombinant HDAC1 protein that carries C-terminal His-FLAG tag was obtained commercially (#10009231; Cayman Chemical, Ann Arbor, MI). The assay was conducted according to the manufacturer’s instruction. Briefly, HDAC1 was diluted (1/10,000) in the HDAC-Glo buffer and was mixed with an equal volume of the buffer containing a test chemical at desired concentrations. After 30 min of incubation at 37 °C, luminescence was measured using the Gene Light 55 Luminometer (Microtech, Chiba, Japan).
2.6 |. RA signaling reporter assay
We previously constructed the RA signaling reporter plasmid RARE-Luc, which contains the firefly luciferase located downstream of the basal promoter with RAR and RXR binding sites (Li & Marikawa, 2016). P19C5 cells were transfected using Lipofectamine 2000 (LifeTechnologies) with RARE-Luc and pRL-TK (Promega), the latter of which encodes Renilla luciferase, to normalize transfection efficiency. The luciferase assay was conducted using the Dual-Luciferase Reporter Assay System (Promega) with Gene Light 55 Luminometer, according to the manufacturer’s instruction.
2.7 |. Statistical analyses
All adverse morphogenetic effects shown in the present study were statistically significant (p < .01), based on two-sample t test that was performed between control and chemical-treated groups. For gene expression analyses, two-sample t test was performed between control and chemical-treated groups to determine significant changes in relative expression levels (p < .05).
3 |. RESULTS
3.1 |. Methoxyacetic acid impairs morphogenesis of mouse gastruloids at teratogenic concentrations
We examined morphological parameters, namely relative area and relative aspect ratio, of mouse P19C5 gastruloids after 4days of culture with various concentrations of MAA. While both parameters were decreased by MAA exposures in a concentration-dependent manner (Figure 2a,b), relative aspect ratio, which represents the extent of axial elongation, was more sensitively affected. For example, at 2 and 4mM, the relative aspect ratio was reduced by 49 and 63%, respectively, whereas the relative area was reduced only by 5 and 14%, respectively (Figure 2b). Note that these concentrations are close to the maternal plasma level of MAA (Cmax=5mM) that causes embryo malformations (Daston et al., 2014; Sleet, Welsch, Myers & Marr, 1996). By contrast, morphogenesis was not impaired by methoxyethanol, a nonteratogenic precursor of MAA, even at much higher concentrations (50 to 200mM) than MAA (Figure 2c). Thus, P19C5 gastruloid morphogenesis was sensitively and selectively affected by MAA in a manner consistent with in vivo situations.
FIGURE 2.
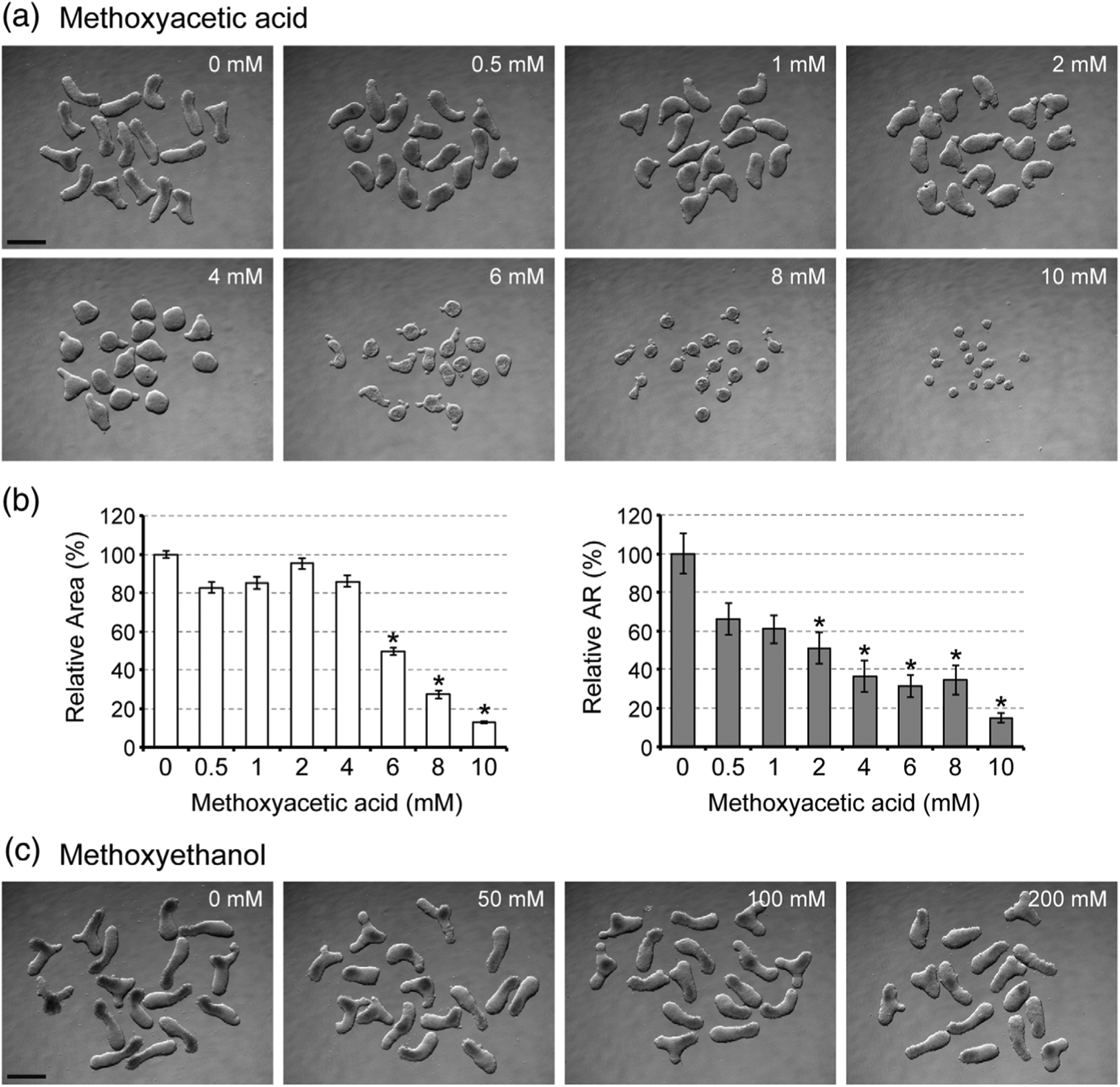
Axial elongation morphogenesis of mouse gastruloids is diminished by methoxyacetic acid (MAA). (a) Images of Day 4 gastruloids that were treated with MAA at different concentrations. (b) Morphometric parameters of MAA-treated gastruloids. Graphs show the averages of relative area (left) and relative aspect ratio (AR; right) with error bars of 95% confidence interval (n = 48). Asterisks indicate adverse impacts, which are defined as reduction in relative area by >20% or relative AR by >40% compared to the control, which is set as 100%. All adverse impacts were statistically significant (p < .01). (c) Images of Day 4 gastruloids that were treated with methoxyethanol at different concentrations. Scale bars = 500 μm
3.2 |. Methoxyacetic acid alters expression profiles of developmental regulator genes
To gain insights into the molecular mechanisms of MAA teratogenicity, we examined gene expression profiles in gastruloids that were treated with MAA at 4 mM. This concentration was chosen because it is close to the in vivo teratogenic concentration (Daston et al., 2014; Sleet, Welsch, Myers & Marr, 1996), and also it robustly inhibited gastruloid elongation (Figure 2a,b). Transcript levels of specific developmental regulator genes (their molecular characteristics, functions, and expression patterns in the normal mouse embryo are summarized in Table 2) were analyzed by quantitative RT-PCR over the course of 4 days of culture. The expressions of pluripotency maintenance factors (Pou5f1 and Nanog) in MAA-treated gastruloids were downregulated to the basal level by Day 2 of culture in a manner similar to control gastruloids (Figure 3). By contrast, the expression profiles of the other genes were significantly altered by MAA. Namely, the Day 1 peak expressions of genes that are involved in the initiation of gastrulation (Wnt3, Brachyury, and Mixl1), the Day 2 peak expressions of genes associated with continued gastrulation and caudal patterning (Snai1, Msgn1, Tbx6, Notch1, and Lfng), and the Day 3 peak expression of the gene essential for segmentation along the body axis (Meox1), were all significantly repressed by MAA (Figure 3). It is likely that the diminished expressions of these developmental regulators contributed to the impairment of axial elongation in MAA-treated gastruloids.
FIGURE 3.
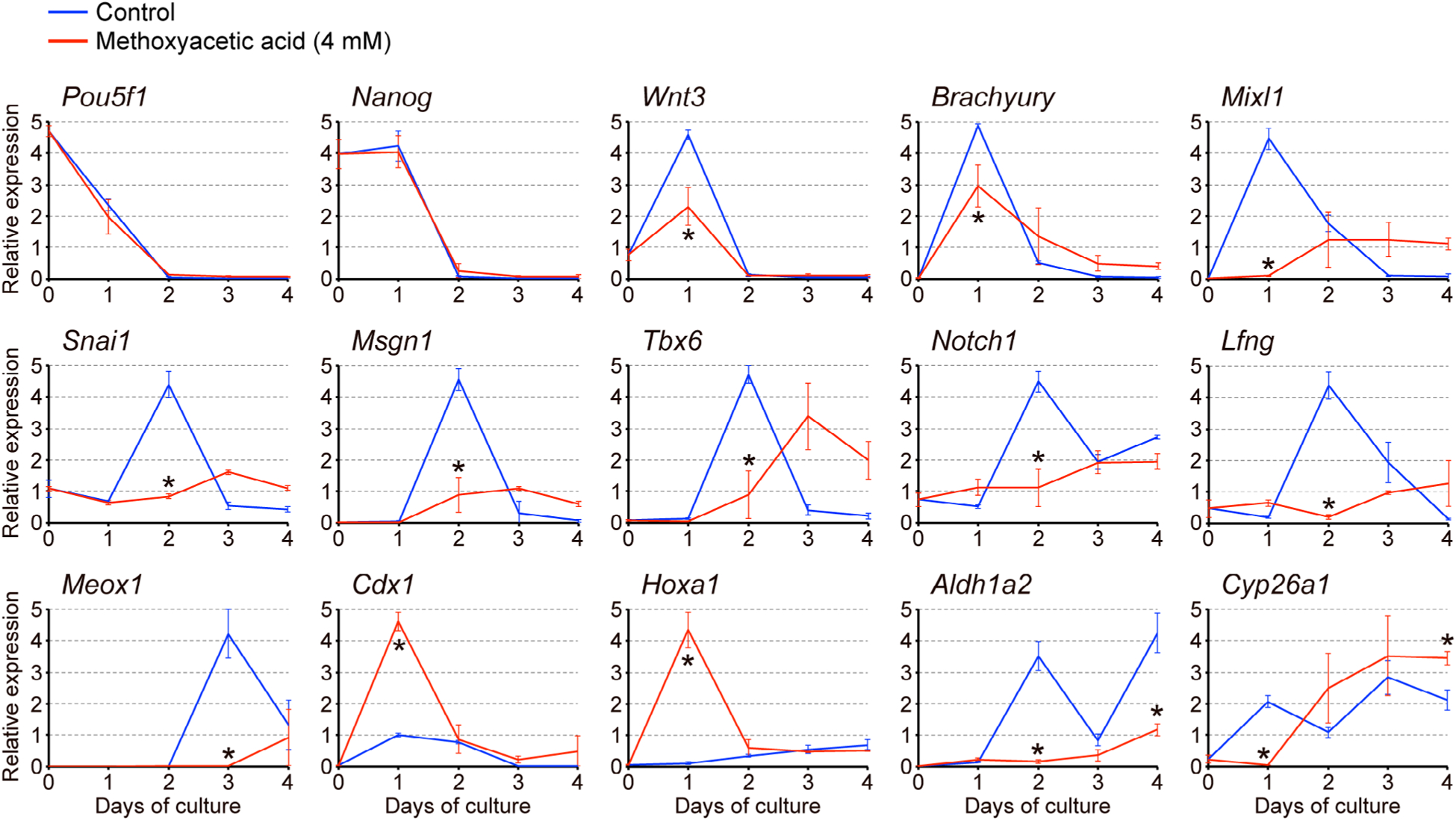
Methoxyacetic acid (MAA) alters gene expression profiles in gastruloids. Temporal expression profiles of developmental regulator genes in gastruloids over a 4-day culture period, as determined by quantitative RT-PCR analyses. Horizontal axes represent days of culture, whereas vertical axes represent relative expression levels in arbitrary units, which were normalized against Actb as a representative housekeeping gene. Blue and red lines correspond to the relative expression levels (mean ± SD; n = 3) in control gastruloids and those treated with 4 mM MAA, respectively. Each biological replicate was a pool of nine samples: Day 0, control gastruloids at Days 1 to 4, and MAA-treated gastruloids at Days 1 to 4, all of which were prepared from the same cell suspension. Asterisks indicate a significant reduction or increase (p < .05) in mean relative expression levels due to MAA on a given day of culture
However, we found that the expressions of Cdx1 and Hoxa1, both of which are regulators of anterior–posterior axial patterning, were markedly elevated by MAA at Day 1 (Figure 3). Because these two genes are well-known transcriptional targets of RA signaling (Houle, Sylvestre, & Lohnes, 2003; Langston & Gudas, 1992), we further examined the expressions of Aldh1a2 and Cyp26a1, which encode the key regulators of RA metabolism through biosynthesis and degradation, respectively (Table 2). Their expression patterns were significantly changed by MAA: the Aldh1a2 level remained low at Days 2 and 4, whereas the Cyp26a1 level was low at Day 1 but significantly increased by Day 4 (Figure 3). These results raise the possibility that MAA alters RA signaling in gastruloids.
3.3 |. Methoxyacetic acid affects gene expressions and axial morphogenesis in a retinoic acid signaling-dependent manner
As shown above, MAA markedly elevated the expression of Cdx1 and Hoxa1 by Day 1 of gastruloid development, which took place under the differentiating condition (i.e., three-dimensional cell aggregation in the presence of 1% DMSO). To test whether MAA can also elevate these RA signaling target genes under a nondifferentiating condition, monolayered cells (without DMSO) were treated with MAA for 1 day and analyzed by quantitative RT-PCR. Cells that were treated with RA at physiological concentrations (5 to 20 nM) exhibited robust up-regulations of Cdx1 and Hoxa1, confirming that these genes are indeed transcriptional targets of RA signaling in P19C5 cells (Figure 4a). MAA also upregulated Cdx1 and Hoxa1 in a dose-dependent manner. Notably, upregulations of Cdx1 and Hoxa1 by MAA (4 mM) were significantly blunted by co-treatment with BMS493, a pharmacological inhibitor of RA receptors (Figure 4b). These results suggest that MAA activates transcriptions of Cdx1 and Hoxa1 in a RA signaling-dependent manner.
FIGURE 4.
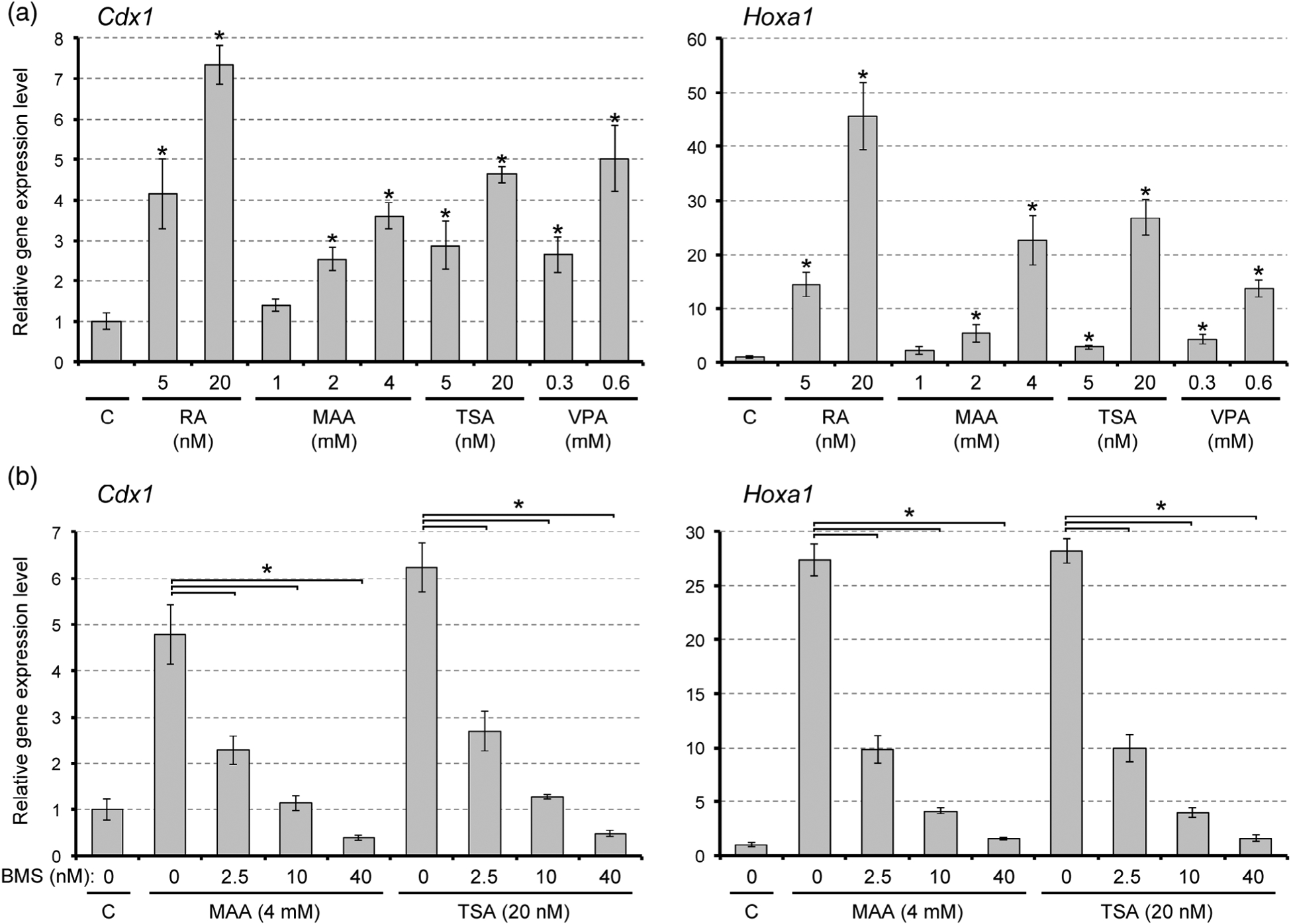
Methoxyacetic acid (MAA) and inhibitors of histone deacetylase up-regulate Cdx1 and Hoxa1 expressions in a retinoic acid (RA) signaling-dependent manner. Vertical axis represents relative gene expression levels in monolayer culture, as measured by quantitative RT-PCR. Graphs show the relative expression levels (mean ± SD; n = 3). The control level (C) is set as one. (a) Expression levels after treatment with RA, MAA, trichostatin A (TSA), or valproic acid (VPA) for 1 day. Asterisks indicate a significant increase by a given treatment compared with the control (p < .01). (b) Expression levels after co-treatment with BMS493 (BMS) plus MAA or TSA for 1 day. Asterisks indicate a significant difference between the two groups specified by each horizontal line (p < .01)
To test whether the morphogenetic effect of MAA is also dependent on RA signaling, we co-treated gastruloids with MAA and BMS493. In this experiment, we limited compound treatment only to the first 2 days of gastruloid culture (Figure 5a), because our previous study has shown that BMS493 is detrimental to axial elongation when applied during the last 2 days of culture (Li & Marikawa, 2016). With this treatment regimen, MAA alone robustly impaired axial elongation of gastruloids (Figure 5b). However, when MAA and BMS493 were administered together, gastruloids exhibited significant elongation (Figure 5b). Relative aspect ratio of gastruloids that were co-treated with MAA plus BMS493 was significantly higher than those treated with MAA alone (Figure 5c). Treatment with BMS493 alone had no apparent impact on gastruloid morphology (Figure 5b,c), consistent with our previous study (Li & Marikawa, 2016). These results suggest that MAA diminishes axial elongation of gastruloids in a RA signaling-dependent manner.
FIGURE 5.
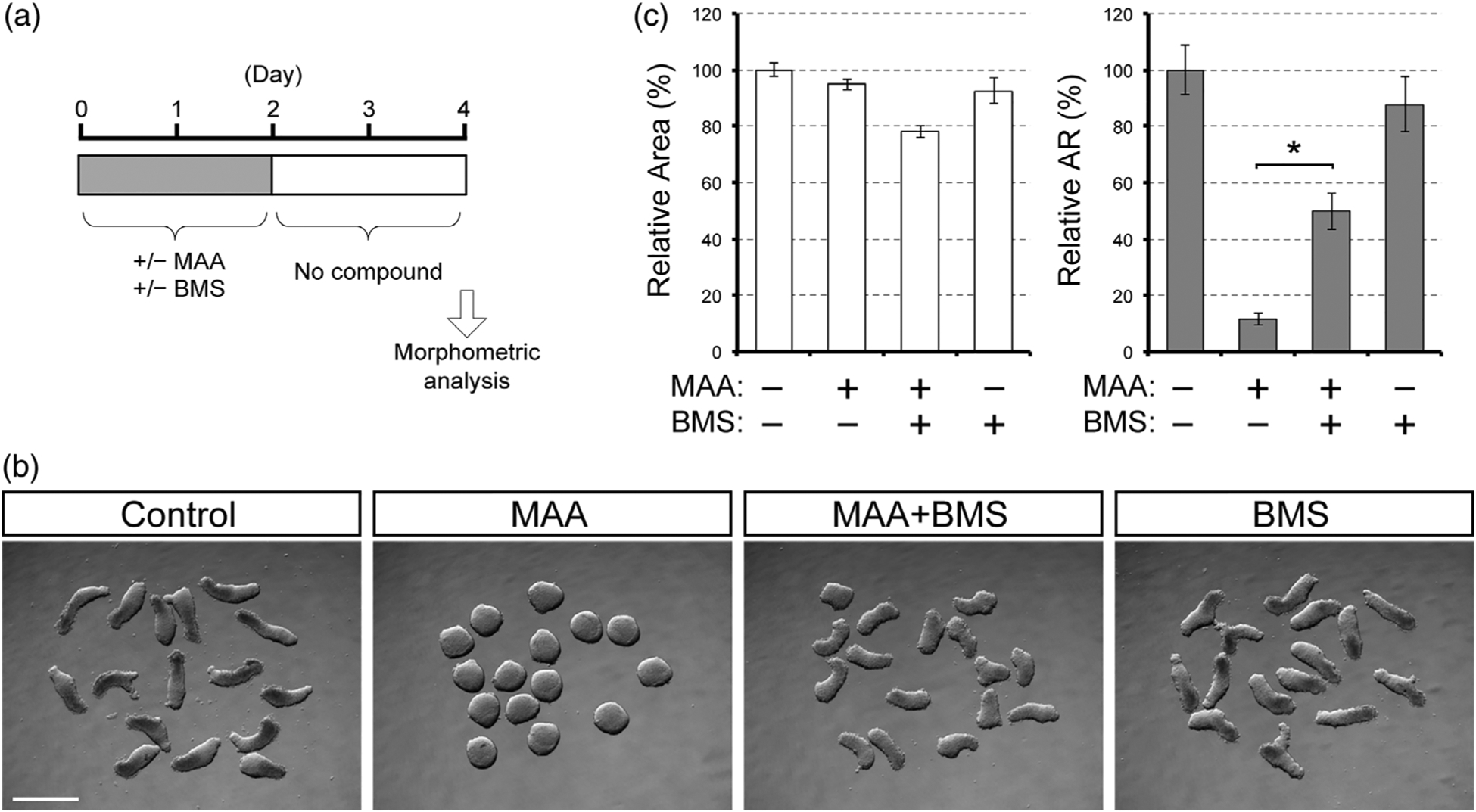
The morphogenetic effect of methoxyacetic acid (MAA) is partly alleviated by the suppression of retinoic acid signaling. (a) A regimen for gastruloid treatment with MAA (4 mM) and BMS493 (BMS, 10 nM). (b) Images of Day 4 gastruloids. Scale bar = 500 μm. (c) Morphometric parameters of treated gastruloids. Graphs show the averages of relative area (left) and relative aspect ratio (AR; right) with error bars of 95% confidence interval (n = 48). Asterisks indicate a significant difference (p < .01) between the MAA and MAA + BMS groups
3.4 |. Methoxyacetic acid acts as an inhibitor of histone deacetylase
The morphogenetic and molecular effects of MAA on gastruloids appear to be similar to those of valproic acid (VPA), because VPA diminishes axial elongation and upregulates Cdx1 and Hoxa1 in a RA signaling-dependent manner (Li & Marikawa, 2016). VPA is an anti-epileptic drug, and is also a well-known teratogen in human and rodents (Jentink et al., 2010; Tomson et al., 2011). The teratogenic action of VPA is thought to be mediated through the inhibition of histone deacetylase (HDAC) (Phiel et al., 2001). This raises the possibility that MAA also inhibits HDAC to cause teratogenic effects.
To directly test whether MAA acts as a HDAC inhibitor, we performed a cell-free enzyme assay, in which the enzymatic activity of purified HDAC1 protein was measured in the presence of MAA or other compounds. HDAC1 activity was diminished by MAA in a dose-dependent manner, and the activity at 4 mM of MAA was about half of the control level (Figure 6a). By contrast, ME, a nonteratogenic precursor of MAA, did not reduce the HDAC1 activity even at 10 mM (Figure 6a). In this assay, HDAC1 activity was also reduced by trichostatin A (TSA), a potent pharmacological inhibitor of HDAC, as well as VPA, in a dose-dependent manner (Figure 6a). Thus, MAA directly inhibited HDAC1 at the teratogenic concentrations.
FIGURE 6.
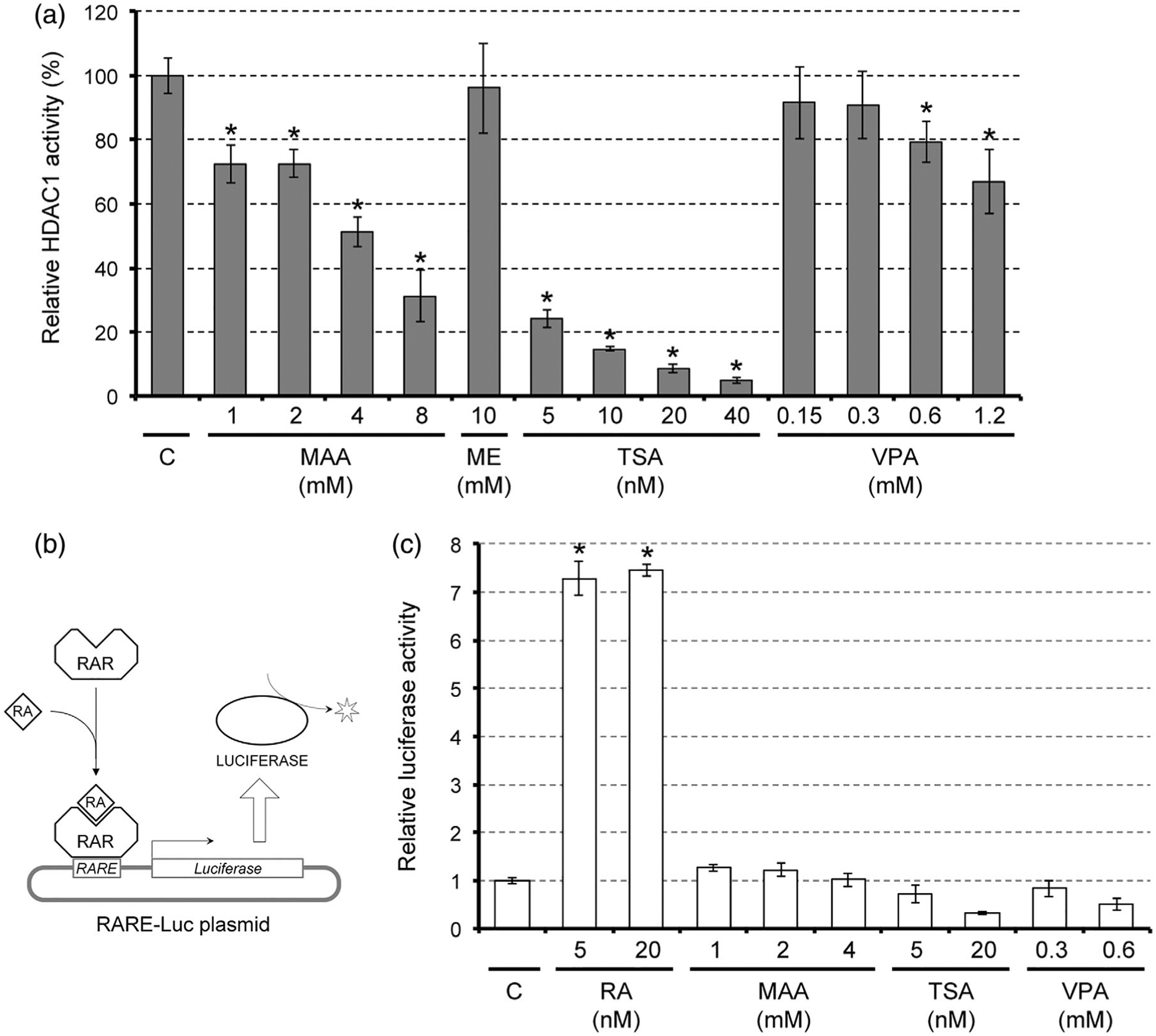
Impact of methoxyacetic acid (MAA) on histone deacetylase (HDAC) activity and retinoic acid (RA) signaling. (a) HDAC activity of purified human HDAC1 protein that was incubated with MAA, methoxyethanol (ME), trichostatin A (TSA), or valproic acid (VPA). Asterisks indicate a significant decrease (p < .05; n = 3) compared to the control (c), which is set as 100%. (b) A schematic diagram, depicting how the RA signaling reporter assay using the RARE-Luc plasmid works. Upon binding of RA, the retinoic acid receptor (RAR) on the RA response element (RARE) in the plasmid activates expression of luciferase, which is then detected using a luminescent substrate. (c) Measurement of RA signaling using the RARE-Luc reporter plasmid in monolayer cells that were treated with chemicals. Graph shows the relative luciferase activity from RARE-Luc (mean ± SD; n = 3). Asterisks indicate a significant increase (p < .01; n = 3) compared with the control (C), which is set as one
The previous study in mouse embryonic stem cells has shown that the inhibition or knockdown of HDAC increases the levels of histone acetylation at the enhancer regions of RA target genes and causes an elevation in their transcriptions (Urvalek & Gudas, 2014). Similarly, treatment of monolayered P19C5 cells with HDAC inhibitors, such as TSA and VPA, upregulated Cdx1 and Hoxa1 in a RA signaling-dependent manner (Figure 4a,b). It is possible that HDAC inhibitors upregulate RA signaling targets by sensitizing the enhancers to the basal level of RA, rather than by increasing the amount of RA itself. To test this possibility, the level of RA signaling was measured using the reporter plasmid RARE-Luc (Figure 6b) in monolayered cells that were treated with HDAC inhibitors, including MAA. The reporter signal was robustly increased by the addition of RA at physiological concentrations, validating the effectiveness of the reporter assay (Figure 6c). However, the reporter signals were not elevated by any of the HDAC inhibitors even at the concentrations that up-regulated Cdx1 and Hoxa1 (Figure 6c). This suggests that HDAC inhibitors, including MAA, do not increase the amount of RA while enhancing the transcription of RA signaling target genes.
4 |. DISCUSSION
In the present study, teratogenic actions of MAA were investigated using mouse gastruloids. Gastruloids are three-dimensional aggregates of pluripotent stem cells that recapitulate key features of gastrulating embryos, and have been employed as in vitro models to investigate molecular mechanisms of embryo patterning (Beccari et al., 2018; Li & Marikawa, 2015; Turner et al., 2017; van den Brink et al., 2014; van den Brink et al., 2020) and to analyze embryotoxic chemical exposures (Kim & Marikawa, 2018; Lau & Marikawa, 2014; Li & Marikawa, 2016; Warkus et al., 2016; Warkus & Marikawa, 2017, 2018; Yuan & Marikawa, 2017). Here, we demonstrated that MAA inhibited gastruloid elongation morphogenesis at the in vivo teratogenic concentrations and that the morphogenetic impact of MAA was partly due to excessive expression of RA signaling target genes. During embryogenesis, the level of RA signaling is spatially and temporally controlled by the coordinated actions of regulatory enzymes, such as ALDH1A2 and CYP26A1, which is pivotal for axial patterning of neural and mesodermal tissues (Piersma, Hessel, & Staal, 2017). As a result, embryo malformations can be induced by disturbance in RA signaling, such as insufficient activation due to vitamin A deficiency or excessive activation due to intake of certain dermatologic medications, including tretinoin (all-trans retinoic acid), isotretinoin (13-cis retinoic acid), and acitretin (Zasada & Budzisz, 2019). Disturbance in RA signaling may be one of the common mechanisms that are linked to the teratogenic actions of various chemical exposures (Piersma et al., 2017). Accordingly, an adverse outcome pathway framework has been proposed for neural tube and axial defects based on RA signaling (Tonk, Pennings, & Piersma, 2015). Morphogenesis of P19C5 gastruloids is sensitively affected by disturbance in RA signaling caused by exposure to all-trans retinoic acid, acitretin, or BMS493 (Li & Marikawa, 2015; Warkus et al., 2016; Warkus & Marikawa, 2017). Thus, the gastruloid may serve as an effective in vitro assay tool to detect chemical exposures that alter RA signaling to cause teratogenesis.
ALDH1A2 and CYP26A1 regulate the amount of RA in an opposing manner: the former mediates biosynthesis and the latter promotes degradation of RA (Piersma et al., 2017). During gastruloid development, the transcript levels of Aldh1a2 and Cyp26a1 are dynamically changed in a complementary fashion (Li & Marikawa, 2015). Specifically, Aldh1a2 is elevated at Days 2 and 4, whereas Cyp26a1 is elevated at Days 1 and 3. This suggests that the level of RA is dynamically changed during gastruloid development. As shown in the present study, an exposure to MAA significantly altered the temporal expression patterns of Aldh1a2 and Cyp26a1. Most notably, the Day 1 elevation of Cyp26a1 was markedly suppressed by MAA (Figure 3), which may in turn lead to an increase in the RA level. However, such a possible increase in the RA level is unlikely to have contributed substantially to the up-regulation of the RA signaling target genes (Cdx1 and Hoxa1) by MAA treatment. This is because the reporter assay (Figure 6c) suggests that the RA level was not significantly elevated by MAA treatment in spite of the marked upregulation of Cdx1 and Hoxa1. Nonetheless, this does not exclude the possibility that alterations in the RA level may be involved in other adverse effects induced by MAA exposure.
The morphogenetic and molecular effects of MAA on P19C5 gastruloids are similar to those of VPA (Li & Marikawa, 2016), such that they both diminished axial elongation and upregulated the RA signaling target genes. These similarities are likely to be due to their common molecular actions as HDAC inhibitors. Acetylation and deacetylation of histones are mediated by histone acetyltransferases and HDAC, respectively, and play crucial roles in transcriptional regulation (Lehrmann, Pritchard, & Harel-Bellan, 2002; Mai et al., 2005). Generally, inhibition of HDAC increases histone acetylation, which leads to transcriptional up-regulation. In P19C5 gastruloids, transcriptional up-regulation of the RA signaling targets appear to be responsible for the morphogenetic effects of VPA and MAA, because axial elongation was partly restored by co-treatment with BMS493 (Li & Marikawa, 2016; the present study). However, axial elongation was not completely restored (Figure 5), raising the possibility that other mechanisms are also involved. Inhibition of HDAC increases histone acetylation at the genome-wide level (Wang, Yan-Neale, Zeremski, & Cohen, 2004), which is likely to up-regulate various other genes. HDAC inhibition can also increase acetylation of nonhistone proteins, such as p53 (Paradis & Hales, 2015), which plays diverse regulatory roles in cell cycle progression and stem cell maintenance (Solozobova & Blattner, 2011). Furthermore, MAA may exert biochemical effects that are unrelated to HDAC inhibition, such as interference with folate-dependent one-carbon transfer reactions (Welsch, Sleet, & Greene, 1987) and activation of PI3K pathway (Bagchi, Hurst, & Waxman, 2009). The roles of these mechanisms in MAA-induced teratogenesis may be further investigated using gastruloids, because such in vitro systems are more amenable for molecular interrogations.
While the teratogenic potential of MAA is evident from model animal studies, it is not clear whether human embryos are also impaired by MAA exposure in a similar manner. Metabolic precursors of MAA, such as ME and DMEP, are widely distributed as an organic solvent and plasticizer, respectively (Bao et al., 2015; Johanson, 2000). As a result, many people, including women of childbearing age, may be exposed to a high level of MAA. In a workplace with daily exposure of 4.5 ppm ME, which is within the permissible exposure limit, MAA concentration in the urine reaches up to 0.6 mM (Shih, Liou, Chen, & Smith, 2001). An epidemiologic study suggests that exposure to ME is associated with increased risks of spontaneous abortion and subfertility in women (Correa et al., 1996). Nonetheless, it is difficult to ascertain from epidemiologic studies that such reproductive outcomes are due to direct actions of MAA on developing embryos, instead of indirect consequences from maternal toxicity. Because experimentations with actual human embryos are nearly impossible for ethical reasons, alternative models are desired that reflect the genomic constitution and biochemical property of human embryonic cells. Accordingly, human pluripotent stem cell lines, which are either derived from preimplantation embryos or created by nuclear reprogramming of somatic cells, have been explored as in vitro models to investigate teratogenic effects of chemical exposures (Flamier, Singh, & Rasmussen, 2017; Kameoka, Babiarz, Kolaja, & Chiao, 2014; Mayshar, Yanuka, & Benvenisty, 2011; Mehta, Konala, Khanna, & Majumdar, 2008; Palmer et al., 2013; West, Weir, Smith, Donley, & Cezar, 2010; Xing et al., 2017). Recent studies reported elongation morphogenesis in three-dimensional aggregates of human pluripotent stem cells (Libby et al., 2020; Marikawa, Chen, Menor, Deng, & Alarcon, 2020; Ninomiya et al., 2020). Such human cell-based morphogenesis models may be exploited to investigate the teratogenic potential of MAA on human embryos, particularly with respect to the mechanism of action (e.g., whether it upregulates the RA signaling targets) and adverse effect concentrations (e.g., whether it impairs morphogenesis at around 5 mM).
Supplementary Material
ACKNOWLEDGMENT
This work was funded by grants from the Alternatives Research & Development Foundation (ARDF) and the National Institutes of Health (NIH; R03HD088970) to Y. M., and was also supported by the NIH Centers of Biomedical Research Excellence (COBRE) Phase 3 (P30GM131944), which was awarded to the Institute for Biogenesis Research. The authors are grateful to Dr. Vernadeth B. Alarcon for reading the article and providing valuable comments.
Funding information
Alternatives Research and Development Foundation; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: R03HD088970; National Institute of General Medical Sciences, Grant/Award Number: P30GM131944
Footnotes
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- Bagchi G, Hurst CH, & Waxman DJ (2009). Interactions of methoxyacetic acid with androgen receptor. Toxicology and Applied Pharmacology, 238(2), 101–110. 10.1016/j.taap.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Wang M, Ning X, Zhou Y, He Y, Yang J, … Chen B (2015). Phthalate concentrations in personal care products and the cumulative exposure to female adults and infants in Shang-hai. Journal of Toxicology and Environmental Health, Part A, 78 (5), 325–341. 10.1080/15287394.2014.968696 [DOI] [PubMed] [Google Scholar]
- Beccari L, Moris N, Girgin M, Turner DA, Baillie-Johnson P, Cossy AC, … Arias AM (2018). Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature, 562(7726), 272–276. 10.1038/s41586-018-0578-0 [DOI] [PubMed] [Google Scholar]
- Brown NA, Holt D, & Webb M (1984). The teratogenicity of methoxyacetic acid in the rat. Toxicology Letters, 22(1), 93–100. [DOI] [PubMed] [Google Scholar]
- Carver EA, Jiang R, Lan Y, Oram KF, & Gridley T (2001). The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Molecular and Cellular Biology, 21 (23), 8184–8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DL, Agulnik I, Hancock S, Silver LM, & Papaioannou VE (1996). Tbx6, a mouse T-box gene implicated in paraxial mesoderm formation at gastrulation. Developmental Biology, 180(2), 534–542. [DOI] [PubMed] [Google Scholar]
- Clarke DO, Duignan JM, & Welsch F (1992). 2-Methoxyacetic acid dosimetry-teratogenicity relationships in CD-1 mice exposed to 2-methoxyethanol. Toxicology and Applied Pharmacology, 114(1), 77–87. [DOI] [PubMed] [Google Scholar]
- Correa A, Gray RH, Cohen R, Rothman N, Shah F, Seacat H, & Corn M (1996). Ethylene glycol ethers and risks of spontaneous abortion and subfertility. American Journal of Epidemiology, 143(7), 707–717. [DOI] [PubMed] [Google Scholar]
- Daston GP, Beyer BK, Carney EW, Chapin RE, Friedman JM, Piersma AH, … Scialli AR (2014). Exposure-based validation list for developmental toxicity screening assays. Birth Defects Research. Part B, Developmental and Reproductive Toxicology, 101(6), 423–428. 10.1002/bdrb.21132 [DOI] [PubMed] [Google Scholar]
- Dayan C, & Hales BF (2014). Effects of ethylene glycol monomethyl ether and its metabolite, 2-methoxyacetic acid, on organogenesis stage mouse limbs in vitro. Birth Defects Research. Part B, Developmental and Reproductive Toxicology, 101(3), 254–261. 10.1002/bdrb.21108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamier A, Singh S, & Rasmussen TP (2017). A standardized human embryoid body platform for the detection and analysis of teratogens. PLoS ONE, 12(2), e0171101. 10.1371/journal.pone.0171101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AH, Hartley L, Sourris K, Stadler ES, Li R, Stanley EG, … Robb L (2002). Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development, 129(15), 3597–3608. [DOI] [PubMed] [Google Scholar]
- Haselbeck RJ, Hoffmann I, & Duester G (1999). Distinct functions for Aldh1 and Raldh2 in the control of ligand production for embryonic retinoid signaling pathways. Developmental Genetics, 25(4), 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann BG (1991). Expression pattern of the Brachyury gene in whole-mount TWis/TWis mutant embryos. Development, 113 (3), 913–917. [DOI] [PubMed] [Google Scholar]
- Houle M, Sylvestre JR, & Lohnes D (2003). Retinoic acid regulates a subset of Cdx1 function in vivo. Development, 130(26), 6555–6567. [DOI] [PubMed] [Google Scholar]
- Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, & Kopan R (2000). Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature, 405(6789), 966–970. [DOI] [PubMed] [Google Scholar]
- Jansen MS, Nagel SC, Miranda PJ, Lobenhofer EK, Afshari CA, & McDonnell DP (2004). Short-chain fatty acids enhance nuclear receptor activity through mitogen-activated protein kinase activation and histone deacetylase inhibition. Proceedings of the National Academy of Sciences of the United States of America, 101(18), 7199–7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentink J, Loane MA, Dolk H, Barisic I, Garne E, Morris JK, … EUROCAT Antiepileptic Study Working Group. (2010). Valproic acid monotherapy in pregnancy and major congenital malformations. The New England Journal of Medicine, 362(23), 2185–2193. 10.1056/NEJMoa0907328 [DOI] [PubMed] [Google Scholar]
- Johanson G (2000). Toxicity review of ethylene glycol monomethyl ether and its acetate ester. Critical Reviews in Toxicology, 30(3), 307–345. [DOI] [PubMed] [Google Scholar]
- Kameoka S, Babiarz J, Kolaja K, & Chiao E (2014). A high-throughput screen for teratogens using human pluripotent stem cells. Toxicological Sciences, 137(1), 76–90. 10.1093/toxsci/kft239 [DOI] [PubMed] [Google Scholar]
- Kim IQ, & Marikawa Y (2018). Embryoid body test with morphological and molecular endpoints implicates potential developmental toxicity of trans-resveratrol. Toxicology and Applied Pharmacology, 355, 211–225. 10.1016/j.taap.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston AW, & Gudas LJ (1992). Identification of a retinoic acid responsive enhancer 3′ of the murine homeobox gene Hox-1.6. Mechanisms of Development, 38(3), 217–227. [DOI] [PubMed] [Google Scholar]
- Lau CG, & Marikawa Y (2014). Morphology-based mammalian stem cell tests reveal potential developmental toxicity of done-pezil. Molecular Reproduction and Development, 81(11), 994–1008. 10.1002/mrd.22423 [DOI] [PubMed] [Google Scholar]
- Lehrmann H, Pritchard LL, & Harel-Bellan A (2002). Histone acetyltransferases and deacetylases in the control of cell proliferation and differentiation. Advances in Cancer Research, 86, 41–65. [DOI] [PubMed] [Google Scholar]
- Li AS, & Marikawa Y (2015). An in vitro gastrulation model recapitulates the morphogenetic impact of pharmacological inhibitors of developmental signaling pathways. Molecular Reproduction and Development, 82(12), 1015–1036. 10.1002/mrd.22585 [DOI] [PubMed] [Google Scholar]
- Li AS, & Marikawa Y (2016). Adverse effect of valproic acid on an in vitro gastrulation model entails activation of retinoic acid signaling. Reproductive Toxicology, 66, 68–83. 10.1016/j.reprotox.2016.09.015 [DOI] [PubMed] [Google Scholar]
- Libby ARG, Joy DA, Elder NH, Bulger EA, Krakora MZ, Gaylord EA, … McDevitt TC (2020). Axial elongation of caudalized human pluripotent stem cell organoids mimics neural tube development. bioRxiv, 03.05.979732. 10.1101/2020.03.05.979732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U,Behringer RR, & Bradley A (1999). Requirement for Wnt3 in vertebrate axis formation. Nature Genetics, 22(4), 361–365. [DOI] [PubMed] [Google Scholar]
- Mai A, Massa S, Rotili D, Cerbara I, Valente S, Pezzi R, … Ragno R (2005). Histone deacetylation in epigenetics: An attractive target for anticancer therapy. Medical Research Reviews, 25(3), 261–309. [DOI] [PubMed] [Google Scholar]
- Mankoo BS, Skuntz S, Harrigan I, Grigorieva E, Candia A, Wright CV, … Pachnis V (2003). The concerted action of Meox homeobox genes is required upstream of genetic pathways essential for the formation, patterning and differentiation of somites. Development, 130(19), 4655–4664. [DOI] [PubMed] [Google Scholar]
- Marikawa Y, Chen HR, Menor M, Deng Y, & Alarcon VB (2020). Exposure-based assessment of chemical teratogenicity using morphogenetic aggregates of human embryonic stem cells. Reproductive Toxicology, 91, 74–91. 10.1016/j.reprotox.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikawa Y, Tamashiro DA, Fujita TC, & Alarcón VB (2009). Aggregated P19 mouse embryonal carcinoma cells as a simple in vitro model to study the molecular regulations of mesoderm formation and axial elongation morphogenesis. Genesis, 47(2), 93–106. 10.1002/dvg.20473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayshar Y, Yanuka O, & Benvenisty N (2011). Teratogen screening using transcriptome profiling of differentiating human embryonic stem cells. Journal of Cellular and Molecular Medicine, 15(6), 1393–1401. 10.1111/j.1582-4934.2010.01105.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A, Konala VB, Khanna A, & Majumdar AS (2008). Assessment of drug induced developmental toxicity using human embryonic stem cells. Cell Biology International, 32(11), 1412–1424. 10.1016/j.cellbi.2008.08.012 [DOI] [PubMed] [Google Scholar]
- Meyer BI, & Gruss P (1993). Mouse Cdx-1 expression during gastrulation. Development, 117(1), 191–203. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, … Yamanaka S (2003). The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell, 113(5), 631–642. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, … Smith A (1998). Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell, 95(3), 379–391. [DOI] [PubMed] [Google Scholar]
- Ninomiya H, Intoh A, Ishimine H, Onuma Y, Ito Y, Michiue T, … Kato M (2020). Application of a human mesoderm tissue elongation system in vitro derived from human induced pluripotent stem cells to risk assessment for teratogenic chemicals. Chemosphere, 250, 126124. 10.1016/j.chemosphere.2020.126124 [DOI] [PubMed] [Google Scholar]
- Palmer JA, Smith AM, Egnash LA, Conard KR, West PR, Burrier RE, … Kirchner FR (2013). Establishment and assessment of a new human embryonic stem cell-based biomarker assay for developmental toxicity screening. Birth Defects Research. Part B, Developmental and Reproductive Toxicology, 98(4), 343–363. 10.1002/bdrb.21078 [DOI] [PubMed] [Google Scholar]
- Paradis FH, & Hales BF (2015). Valproic acid induces the hyperacetylation of P53, expression of P53 target genes, and markers of the intrinsic apoptotic pathway in mid-organogenesis murine limbs. Birth Defects Research. Part B, Developmental and Reproductive Toxicology, 104(5), 177–183. 10.1002/bdrb.21149 [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, & Klein PS (2001). Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. Journal of Biological Chemistry, 276(39), 36734–36741. [DOI] [PubMed] [Google Scholar]
- Piersma AH, Hessel EV, & Staal YC (2017). Retinoic acid in developmental toxicology: Teratogen, morphogen and biomarker. Reproductive Toxicology, 72, 53–61. 10.1016/j.reprotox.2017.05.014 [DOI] [PubMed] [Google Scholar]
- Ritter EJ, Scott WJ Jr., Randall JL, & Ritter JM (1985). Teratogenicity of dimethoxyethyl phthalate and its metabolites methoxyethanol and methoxyacetic acid in the rat. Teratology, 32(1), 25–31. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Meno C, Fujii H, Nishino J, Shiratori H, Saijoh Y, … Hamada H (2001). The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes & Development, 15(2), 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardein JL, & Macina OT (2006). Human developmental toxicants: Aspects of toxicology and chemistry. Boca Raton, FL: CRC Press. [Google Scholar]
- Scott WJ, Fradkin R, Wittfoht W, & Nau H (1989). Teratologic potential of 2-methoxyethanol and transplacental distribution of its metabolite, 2-methoxyacetic acid, in non-human primates. Teratology, 39(4), 363–373. [DOI] [PubMed] [Google Scholar]
- Shifley ET, Vanhorn KM, Perez-Balaguer A, Franklin JD, Weinstein M, & Cole SE (2008). Oscillatory lunatic fringe activity is crucial for segmentation of the anterior but not posterior skeleton. Development, 135(5), 899–908. 10.1242/dev.006742 [DOI] [PubMed] [Google Scholar]
- Shih TS, Liou SH, Chen CY, & Smith TJ (2001). Urinary 2-methoxy acetic acid accumulation in response to 2-methoxy ethanol exposure. Archives of Environmental Health, 56(1), 20–25. [DOI] [PubMed] [Google Scholar]
- Sleet RB, Greene JA, & Welsch F (1988). The relationship of embryotoxicity to disposition of 2-methoxyethanol in mice. Toxicology and Applied Pharmacology, 93(2), 195–207. [DOI] [PubMed] [Google Scholar]
- Sleet RB, Welsch F, Myers CB, & Marr MC (1996). Developmental phase specificity and dose-response effects of 2-methoxyethanol in rats. Fundamental and Applied Toxicology, 29(1), 131–139. 10.1006/faat.1996.0014. [DOI] [PubMed] [Google Scholar]
- Solozobova V, & Blattner C (2011). p53 in stem cells. World Journal of Biological Chemistry, 2(9), 202–214. 10.4331/wjbc.v2.i9.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry KK, Elswick BA, Stedman DB, & Welsch F (1994). Developmental phase alters dosimetry-teratogenicity relationship for 2-methoxyethanol in CD-1 mice. Teratology, 49(3), 218–227. [DOI] [PubMed] [Google Scholar]
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Sabers A, … EURAP study group. (2011). Dose-dependent risk of malformations with antiepileptic drugs: An analysis of data from the EURAP epilepsy and pregnancy registry. The Lancet Neurology, 10(7), 609–617. 10.1016/S1474-4422(11)70107-7 [DOI] [PubMed] [Google Scholar]
- Tonk EC, Pennings JL, & Piersma AH (2015). An adverse outcome pathway framework for neural tube and axial defects mediated by modulation of retinoic acid homeostasis. Reproductive Toxicology, 55, 104–113. 10.1016/j.reprotox.2014.10.008 [DOI] [PubMed] [Google Scholar]
- Turner DA, Girgin M, Alonso-Crisostomo L, Trivedi V, Baillie-Johnson P, Glodowski CR, … Arias AM (2017). Anteroposterior polarity and elongation in the absence of extra-embryonic tissues and of spatially localised signalling in gastruloids: Mammalian embryonic organoids. Development, 144(21), 3894–3906. 10.1242/dev.150391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urvalek AM, & Gudas LJ (2014). Retinoic acid and histone deacetylases regulate epigenetic changes in embryonic stem cells. Journal of Biological Chemistry, 289(28), 19519–19530. 10.1074/jbc.M114.556555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink SC, Alemany A, van Batenburg V, Moris N, Blotenburg M, Vivié J, … van Oudenaarden A (2020). Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature. 10.1038/s41586-020-2024-3 [DOI] [PubMed] [Google Scholar]
- van den Brink SC, Baillie-Johnson P, Balayo T, Hadjantonakis AK, Nowotschin S, Turner DA, & Martinez Arias A (2014). Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development, 141(22), 4231–4242. 10.1242/dev.113001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yan-Neale Y, Zeremski M, & Cohen D (2004). Transcription regulation by histone deacetylases. Novartis Foundation Symposia, 259, 238–245 discussion 245–248, 285–288. [PubMed] [Google Scholar]
- Warkus EL, Yuen AA, Lau CG, & Marikawa Y (2016). Use of in vitro morphogenesis of mouse embryoid bodies to assess developmental toxicity of therapeutic drugs contraindicated in pregnancy. Toxicological Sciences, 149(1), 15–30. [DOI] [PubMed] [Google Scholar]
- Warkus ELL, & Marikawa Y (2017). Exposure-based validation of an in vitro gastrulation model for developmental toxicity assays. Toxicological Sciences, 157(1), 235–245. 10.1093/toxsci/kfx034 [DOI] [PubMed] [Google Scholar]
- Warkus ELL, & Marikawa Y (2018). Fluoxetine inhibits canonical Wnt signaling to impair embryoid body morphogenesis: Potential teratogenic mechanisms of a commonly used antidepressant. Toxicological Sciences, 165(2), 372–388. 10.1093/toxsci/kfy143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellik DM (2009). Hox genes and vertebrate axial pattern. Current Topics in Developmental Biology, 88, 257–278. 10.1016/S0070-2153(09)88009-5 [DOI] [PubMed] [Google Scholar]
- Welsch F, Sleet RB, & Greene JA (1987). Attenuation of 2-methoxyethanol and methoxyacetic acid-induced digit malformations in mice by simple physiological compounds: Implications for the role of further metabolism of methoxyacetic acid in developmental toxicity. Journal of Biochemical Toxicology, 2, 225–240. [DOI] [PubMed] [Google Scholar]
- West PR, Weir AM, Smith AM, Donley EL, & Cezar GG (2010). Predicting human developmental toxicity of pharmaceuticals using human embryonic stem cells and metabolomics. Toxicology and Applied Pharmacology, 247(1), 18–27. 10.1016/j.taap.2010.05.007 [DOI] [PubMed] [Google Scholar]
- Xing J, Cao Y, Yu Y, Li H, Song Z, & Yu H (2017). In vitro micropatterned human pluripotent stem cell test (μP-hPST) for morphometric-based teratogen screening. Scientific Reports, 7(1), 8491. 10.1038/s41598-017-09178-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemoto J, Brown NA, & Webb M (1984). Effects of dimethoxyethyl pthalate, monomethoxy-ethyl phthalate, 2-methoxyenthanol, and methoxyacetic acid on post implantation rat embryos in culture. Toxicology Letters, 21, 97–102. [DOI] [PubMed] [Google Scholar]
- Yoon JK, & Wold B (2000). The bHLH regulator pMesogenin1 is required for maturation and segmentation of paraxial mesoderm. Genes & Development, 14(24), 3204–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CJ, & Marikawa Y (2017). Developmental toxicity assessment of common excipients using a stem cell-based in vitro morphogenesis model. Food and Chemical Toxicology, 109(Pt 1), 376–385. 10.1016/j.fct.2017.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasada M, & Budzisz E (2019). Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Advances in Dermatology and Allergology, 36(4), 392–397. 10.5114/ada.2019.87443 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


