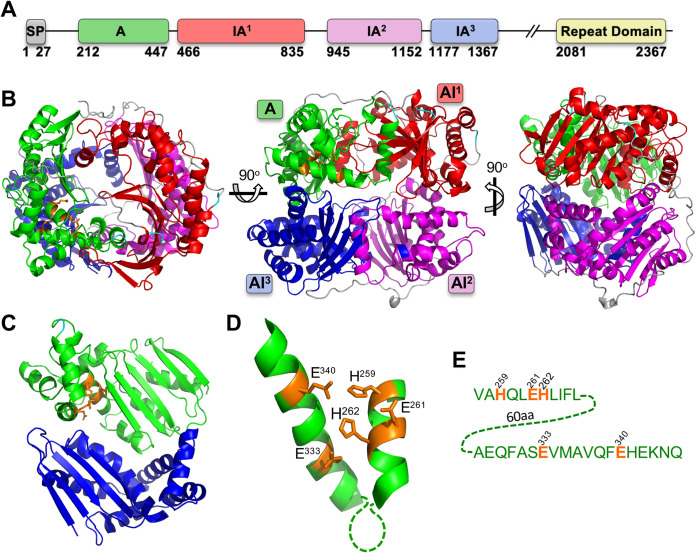
FIG 1.
Structural features of TLN4. (A) Domain structure of TLN4 illustrating its possession of a signal peptide (SP) and, based on the IDE structure, a single active domain (domain A), 3 inactive domains (IA1-3), and a C-terminal repeat domain. Note that a largely featureless region between IA3 and the repeat domain is not shown and is instead denoted by //. Numbering indicates amino acid positions and is based on the sequenced cDNA of TLN4 (22). (B) Structural model of TLN4 showing the arrangement of domains into a shell surrounding a central catalytic chamber. Three different views are provided. (C) The A and AI3 domains viewed from the center of the chamber with resides that coordinate zinc binding shown in orange. (D) Zoom-in of the adjacent alpha helices of the A domain showing the residues (orange) that are predicted to coordinate binding to zinc. (E) Sequence of the alpha helices shown in panel D, including residues (orange) that likely mediate binding to zinc as a cofactor for proteolytic activity.