Abstract
Black Americans have increased risk for schizophrenia and other mental illnesses with prenatal origins. Prenatal choline promotes infant brain development and behavioral outcomes, but choline has not been specifically assessed in Black Americans. Pregnant women (N = 183, N = 25 Black Americans) enrolled in a study of prenatal stressors and interactions with prenatal choline. Black American women had lower 16-week gestation plasma choline than Whites. Lower choline was not related to obesity, income, or metabolic genotypes. Pregnant women in rural Uganda have higher choline levels than Black American women. Black Americans’ lower choline was associated with higher hair cortisol, indicative of higher stress. Lower maternal choline was associated with offsprings’ lower gestational age at birth and with decreased auditory P50 inhibition, a marker of inhibitory neuron development. Behavioral development was assessed on the Infant Behavior Questionnaire-R-SF (IBQ-R) at 3 months. Lower Black American maternal gestational choline was associated with lower infant IBQ-R Orienting/Regulation, indicating decreased attention and relation to caregivers. Additional evidence for developmental effects of choline in Black Americans comes from a randomized clinical trial of gestational phosphatidylcholine supplementation versus placebo that included 15 Black Americans. Phosphatidylcholine increased gestational age at birth and newborn P50 inhibition and decreased Social Withdrawn and Attention problems at 40 months of age in Black Americans’ offspring compared to placebo. Inhibitory and behavioral deficits associated with lower prenatal choline in offspring of Black American women indicate potential developmental predispositions to later mental illnesses that might be ameliorated by prenatal choline or phosphatidylcholine supplementation.
Keywords: phosphatidylcholine, infant preterm, fetal development, African American, child development, schizophrenia
Introduction
Higher rates of schizophrenia, psychotic spectrum disorders, and sub-threshold symptoms of psychosis and mania have been reported in young adults from under-represented groups, including Black Americans, compared to Whites.1,2 Attention deficit disorder is diagnosed significantly more frequently in Black American children from 3 to 17 years of age, compared to Hispanic and White children.3 Autism spectrum disorder rates in Black Americans are also rising above Whites.4 Prenatal maternal infection, nutritional deficiencies, and depression have been associated with increased risk of these mental illnesses.5–8 These factors adversely affect fetal brain development, thereby increasing the risk for later mental illness.9,10 Early developmental signs of illness can appear in infancy.11
Prenatal depression in Black American women and its effects on cortisol and inflammation have complex relationships to systemic racism, neighborhood environment, partner relationships, and financial insecurity.12–18 One concomitant of their maternal depression is lower gestational age at birth, which has enduring consequences for child development.16–19 In addition, Black American mothers are likely to have nutritional deficiencies. Choline was specifically analyzed by periodic dietary recall in one study of Black American women.20 Mean intake in early pregnancy, <22 weeks gestation, was 318 mg/d (SD 68). No woman reached the current US Food and Drug Administration recommended amount.21 Early offspring developmental issues linked to prenatal stress and nutrition can become significant problems when Black American children enter school.22
Prenatal vitamins and folic acid supplements are already widely adopted in prenatal care. The American Medical Association has requested that evidence-based amount of choline be added to prenatal formulations.23 Choline has multiple roles in development, including cell membrane phospholipid synthesis, one-carbon metabolism and methylation, and agonism at cholinergic receptors, before their innervation by acetylcholinergic synapses.24,25 Increased maternal dietary choline and choline supplements have been associated with improved cognitive ability, attention, and social behavior in children up to 7 years old.26–30
This study investigated how prenatal maternal choline and other prenatal vitamins interact with antenatal depression and cortisol levels as a biomarker of the stress response in Black American women and possibly diminish adverse childhood outcomes. A diverse cohort of women was recruited from prenatal clinic admissions in a metropolitan safety-net hospital that serves vulnerable women for a study designed to examine a wide range of prenatal influences on fetal brain development of cerebral inhibition and early childhood behavior. Effects of maternal infection, including respiratory coronaviruses, cannabis use, and inflammation, and their interaction with maternal choline plasma concentration in the cohort of all ancestries have been previously reported.31–34
To distinguish prenatal developmental issues from postnatal factors, it is desirable to assess newborns as soon as possible after birth, despite their limited behavioral repertoire. Inhibition of the cerebral P50 evoked response to a repeated auditory stimulus is a neurophysiological phenotype associated with schizophrenia, bipolar disorder, attention deficit disorder, and autism spectrum disorder.35–37 Inhibition of P50 responses can be recorded in 1-month-old-newborns and used to gauge prenatal effects on central nervous system development.38 We and another group have shown that infants of mothers with schizophrenia spectrum illnesses have diminished P50 inhibition.39,40 We have also found diminished P50 inhibition in newborns of infected mothers, mothers who use cannabis and nicotine, and depressed mothers, all factors potentially associated with the offsprings’ later mental illness.31–34,39
P50 inhibition is likely to reflect brain development between the first and second trimester of gestation. This period is also when maternal infection has been identified epidemiologically as a risk factor for later schizophrenia.7 The hippocampus is a source for the P50 wave of the human cerebral auditory-evoked response.41 Its analog in rodent models is generated by hippocampal pyramidal neurons, and its inhibition in response to repeated sounds involves activation of hippocampal inhibitory interneurons.42 Many of these interneurons develop in human fetuses between 12 and 20 weeks in gestation.43,44 Choline affects the development of inhibition of the P50 cerebral evoked potential via activation of α7-nicotinic cholinergic receptors that facilitate the conversion of the chloride transporter to the mature KCCN form, which permits GABA-sensitive chloride channels to be inhibitory.45 The conversion is not complete in the post mortem brain tissue of persons with schizophrenia.46
The development of inhibitory interneurons, including the synthesis of KCCN-type chloride transporters, occurs when fetal choline levels are at their lowest point in pregnancy. Choline is concentrated from maternal plasma into the amniotic fluid by transporters in the placenta.24 Maternal choline plasma concentrations are lowest early in pregnancy.47 Later in pregnancy, the mother’s increasing estrogen increases the expression of PEMT, the gene that produces Phosphatidylethanolamine N-methyltransferase, the enzyme responsible for her endogenous synthesis of choline.48 Choline plasma concentration thus rises throughout pregnancy, peaking in the third trimester. However, maternal choline concentration at 16 weeks is potentially a critical variable in fetal brain development, because of the early requirement of choline for fetal brain neuronal development before peak maternal PEMT expression and choline synthesis have increased maternal choline concentrations.
Effects of fetal brain development extend past the newborn period. In this cohort, we previously reported that higher newborn P50S2μV, indicative of poorer P50 inhibition, was associated with behavioral differences at 3 months of age, as assessed by the Infant Behavior Questionnaire-R/SF (IBQ-R).31 The IBQ-R Orienting/Regulation Index was particularly affected. Infants with higher P50S2μV and associated lower IBQ-R Orienting/Regulation were less soothable, harder to engage, had lower duration of attention, and expressed less enjoyment from cuddling with their mothers. In an earlier clinical trial of phosphatidylcholine supplementation, diminished P50 inhibition was associated with childhood behavioral problems through 4 years of age.28
For the present study, we examined differences in 16-week choline plasma concentrations between Black American, Native American, and White women. We hypothesized maternal nutrition, income, choline metabolic genotype, ancestral background, and stress as possible associated factors for lower choline levels in Black Americans. We hypothesized that lower choline levels would affect the outcomes of gestation, beginning with gestational age at birth and extending to early brain development. We specifically hypothesized that if Black American women have lower 16-week plasma choline compared to other women, their newborns compared to others would have higher amplitude P50 responses to the repeated sound, indicating poorer development of inhibition. As the offspring developed into childhood, we hypothesized that lower choline levels and poorer newborn inhibition of P50 would be associated with poorer IBQ-R Orienting/Regulation and other behavioral measures in later childhood.
Methods
Subjects
For the study of maternal choline plasma concentration, 183 unrelated women, nulliparous or multiparous, were enrolled from the Denver Health prenatal clinic at 14–16 weeks gestation from July 2013 until July 2016. Mothers and fathers were asked to characterize their ancestry, race, and ethnicity as they felt appropriate, without pre-defined categories. Twenty-five reported Black, African American, or multiethnic, including these ancestries. Hispanic ethnicity was reported by members of both Black American and White groups. Enrolling staff had diverse ethnicities. Gestational age was established by first ultrasound.49
For a previously reported randomized trial of phosphatidylcholine supplementation, 100 women were enrolled; 15 were Black Americans.28 The Colorado Multiple Institution Review Board approved both studies under the Common Rule; all mothers, and fathers if available, gave informed consent.
Women in Uganda (N = 166) were enrolled from the Gulu regional referral hospital in rural northern Uganda at 18–25 weeks gestation, established by last menstrual period. The study was approved by the Makerere University College of Health Sciences School of Medicine Research Ethics Committee and the Uganda National Council of Science and Technology.
Assessments
Previously described methods were used for assessment of maternal mood and stressors, cortisol and choline concentrations, newborn P50 recording, and infant and childhood behavior (supplementary methods).
Statistical Analyses
General linear models and multiple regression were used for analyses. Principal outcomes were established from previous studies: gestational age at birth, P50S2μV, IBQ-R Orienting/Regulation, and Child Behavior Checklist (CBCL) 1½–5 years Withdrawn and Attention Problems.28,31 Potential covariates were identified from comparisons of Black Americans and Whites (table 1). Maternal Center for Epidemiological Studies of Depression (CESD) depression ratings were correlated with both P50S2μV (r = .293, P < .001) and IBQ-R ratings (r = −.169, P = .047) and were a covariate for those analyses. IBQ-R Surgency/Extraversion was a covariate for Orienting/Regulation (r = .645, P = .005) as others have reported in under-represented groups.50 Maternal age and sociodemographic differences were covariates for IBQ-R ratings; they did not significantly affect P50S2μV. Gestational age at birth and at assessments and offspring sex were standard covariates. Prenatal vitamins with folic acid were an additional covariate, to compare effects with choline. Significance criterion was α = 0.05 2-tail.
Table 1.
Differences Between Mothers and Offspring by self-Identified Maternal Ancestrya
| Black American, N = 23 | White, N = 126 | Significance | |||
|---|---|---|---|---|---|
| Maternal and Offspring Parameters | Mean | SD | Mean | SD | t test P |
| Maternal characteristics | |||||
| Maternal age, y | 29.3 | 5.9 | 30.1 | 5.9 | .6 |
| Center for Epidemiological Studies of Depression (CESD) 16 wk gestation | 17.1 | 10.7 | 13.1 | 9.2 | .06 |
| CESD 28 wk gestation | 16.7 | 11.6 | 13.5 | 9.1 | .1 |
| State Trait-Anxiety Inventory-State at 16 wk gestation | 38.3 | 12.0 | 35.2 | 10.7 | .22 |
| Perceived Stress Scale at 16 wk gestation | 24.7 | 8.3 | 23.2 | 8.1 | .42 |
| Maternal education, y | 13.1 | 2.1 | 13.8 | 3/3 | .37 |
| Maternal highest occupation Socio-Economic Index | 42.5 | 15.9 | 47.7 | 21.8 | .28 |
| Maternal pre-pregnancy BMI | 27.7 | 9.0 | 26.7 | 6.0 | .5 |
| Increase in BMI at delivery | 5.0 | 3.2 | 5.2 | 2.8 | .8 |
| Adverse Childhood Experiences (ACE) | 3.59 | 2.1 | 2.31 | 2.4 | .04 |
| Maternal cortisol log10 pg/mg hair 16 wk gestation | 0.93 | 0.47 | 0.74 | 0.41 | .06 |
| Maternal cortisol log10 pg/mg hair 28 wk gestation | 1.20 | 0.51 | 0.84 | 0.41 | .001 |
| Choline plasma μM 16 wk Gestation | 5.48 | 1.40 | 6.58 | 1.90 | .008 |
| Betaine plasma μM 16 wk gestation | 11.05 | 3.44 | 11.75 | 3.70 | .4 |
| Dimethylglycine plasma μM 16 wk gestation | 8.33 | 2.58 | 8.88 | 2.93 | .4 |
| Choline plasma μM 28 wk gestation | 7.97 | 2.20 | 7.19 | 1.90 | .1 |
| Betaine plasma μM 28 wk gestation | 10.54 | 2.29 | 10.70 | 3.43 | .8 |
| Dimethylglycine plasma μM 28 wk gestation | 8.87 | 3.46 | 7.79 | 2.97 | .1 |
| N | % | N | % | Fisher’s exact P | |
| Infection in first trimester, viral or bacterial brought to medical attention or endorsed by mother as moderate to severe | 11 | 48% | 47 | 37% | .9 |
| Obesity pre-pregnancy BMI ≥ 30 | 5 | 22% | 38 | 30% | .9 |
| Alcohol use at 16 wk gestation | 4 | 17% | 14 | 11% | .5 |
| Cannabis use at 16 wk gestation | 2 | 9% | 21 | 17% | .5 |
| Tobacco use at 16 wk Gestation | 1 | 4% | 10 | 8% | .9 |
| Biological father present | 13 | 57% | 94 | 75% | .08 |
| Hispanic | 4 | 17% | 70 | 56% | .001 |
| Antidepressant use | 3 | 13% | 16 | 14% | .9 |
| Pre-eclampsia | 1 | 4% | 13 | 10% | .7 |
| Prenatal vitamins with folate | 19 | 83% | 112 | 89% | .5 |
| Nulliparous | 11 | 48% | 42 | 30% | .1 |
| Gestational diabetes | 2 | 9% | 4 | 3% | .2 |
| Prematurity <37 wk | 7 | 30% | 13 | 10% | .017 |
| Vaginal delivery | 20 | 86% | 87 | 71% | .9 |
| Small for gestational age | 0 | 0% | 0 | 0% | 1.0 |
| Large for gestational age | 2 | 9% | 19 | 15% | .5 |
| Fetal Sex (male) | 9 | 39% | 66 | 52% | .26 |
| Breast feeding N (%) | 21 | 91% | 113 | 90% | .9 |
| Male neonate | Mean | SD | Mean | SD | t test P |
| Gestational age at birth d | 261.9 | 31.4 | 274.8 | 12.3 | .03 |
| Birth weight g | 2774.4 | 924.1 | 3331.1 | 523.2 | .14 |
| Birth head circumference cm | 33.6 | 3.4 | 35.2 | 2.8 | .14 |
| Birth length cm | 47.5 | 5.8 | 49.8 | 4.2 | .3 |
| APGAR 5 min | 8.1 | 1.1 | 8.9 | 0.4 | .11 |
| Female neonate | |||||
| Gestational age at birth, d | 264.9 | 12.1 | 271.0 | 17.4 | .08 |
| Birth weight, g | 2768.8 | 496.2 | 3090.5 | 578.0 | .3 |
| Birth head circumference, cm | 33.2 | 1.4 | 33.9 | 2.0 | .26 |
| Birth length, cm | 47.9 | 2.9 | 49.4 | 3.2 | .16 |
| APGAR 5 min | 8.9 | 0.3 | 8.8 | 0.5 | .5 |
| Newborn Electrophysiological recording 1 mo of age | |||||
| P50S1μV | 1.72 | 1.00 | 1.67 | 0.84 | .8 |
| P50S2μV | 0.99 | 0.54 | 0.77 | 0.46 | .04 |
| Gestational age at P50 recording days | 28.0 | 8.1 | 28.2 | 10.2 | .9 |
| Infant Behavioral Questionnaire-R (SF) 3 mo of age | |||||
| Surgency/extraversion | 4.66 | 1.16 | 4.17 | 1.10 | .07 |
| Negative affectivity | 3.32 | 0.81 | 3.08 | 0.94 | .3 |
| Orienting/regulation | 5.32 | 0.68 | 5.23 | 0.70 | .6 |
| Gestational age at IBQ-R wk | 12.8 | 1.6 | 12.8 | 1.7 | .9 |
Note:aThe samples are women whose newborns completed P50 recordings.
Results
Of the 183 women enrolled, 25 identified themselves as Black, African Americans, or multiethnic ancestry, including Black. By the 16th week of gestation, 162 women completed initial assessment, including plasma choline level, demographic information, and ratings of their mental condition (supplementary figure S1). Twenty-three identified themselves as Black, African American, or multiethnic ancestry, including Black or African American; 13 identified as Native American. The remainder identified as White. Compared with White women, the Black American women had higher self-rating of depression symptoms and adverse childhood experiences, were less likely to have Hispanic heritage, and less likely to be living with the biological father of their child. Prenatal vitamins with folate were used by 19 (83%) of Black American mothers and 112 (89%) of White mothers. Over 90% of Black American and White mothers breast-fed their neonates (table 1).
Differences in Black American and White Maternal Plasma Choline and Possible Factors
Ancestry significantly affected plasma choline, Fdf2,160 = 3.958, P = .021. Post hoc tests found that Black American mean plasma choline 5.48 μM (SD 1.40) was significantly lower than Whites 6.58 μM (SD 1.90), P = .008 (table 1). Women who identified as Black Americans, N = 10, choline 5.38 μM (SD 1.36), or multiethnic Black Americans, N = 13, choline 5.40 μM (SD 1.49), had similar levels; these 2 groups were combined for further analyses. Plasma choline was also measured at 28 weeks gestation and no ancestral group differences were found. Hispanic ethnicity did not affect plasma choline and did not interact with Black and White ancestries (supplementary table S1).
No Effects of Obesity, Lower Household Income, or Metabolic Pathway Genotypes
Maternal obesity, often linked to consumption of calorie-dense, nutrient-poor food, was not significantly different in Black Americans and was not related to plasma choline (table 1, supplementary table S2). In the lowest quartile of household income, Black American women had lower choline than Whites (table 2, supplementary table S3).
Table 2.
Distribution of Mothers in the Denver, Colorado, Metropolitan Area by Income Quartile, Estimated From Household Postal Zip Code
| Household | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|
| Income quartiles 2017 | >$125 000 | $69 000–$125 000 | $35 000–$68 000 | <$35 000 |
| Population distributiona | ||||
| Black American | 3 (13%) | 5 (22%) | 6 (26%) | 9 (39%) |
| White | 32 (25%) | 22 (18%) | 33 (26%) | 39 (31%) |
| Choline plasma concentration 16 wks. gestation mean μM (SE) | ||||
| Black American | 6.18 (1.05) | 5.38 (0.81) | 5.31 (0.68) | 5.42 (0.64)b |
| White | 6.83 (0.32) | 6.46 (0.39) | 6.06 (0.32) | 6.87 (0.29) |
Note:aχ 2df3 = 1.85, P = .6.
bComparison with Whites in Quartile 4, P = .04.
Black American maternal genotypes associated with choline metabolism, particularly PEMT rs3760188 and rs4646343, mirrored the general American population and were significantly different from African genotypes. These genotypes were not associated with differences in plasma choline (supplementary table S4).
Comparison With Women in Uganda
Plasma choline levels obtained during pregnancy from 166 women in rural Uganda were significantly higher than Black American levels, even if the women were HIV positive (table 3).
Table 3.
Choline Concentrations of Ugandan Women Compared With Black American Women
| N | Choline Plasma μM, Mean (SD) | Betaine Plasma μM, mean (SD) | Dimethylglycine Plasma μM, Mean (SD) | |
|---|---|---|---|---|
| Ugandan women mean median 23 wk gestation | 144 | 9.19 (4.82)a | 12.75 (6.41) | 5.67 (2.41)b |
| Ugandan women with HIV | 22 | 9.07 (4.72) | 12.76 (8.20) | 5.20 (2.11) |
| Black American women 16- to 28-wk pregnancy mean | 23 | 6.51 (1.70) | 10.76 (2.13) | 8.40 (2.45) |
Note:aComparison with Black American women, P < .01.
bComparison with Black American women, P < .001.
Maternal Cortisol Association With Lower Maternal Choline in Black Americans
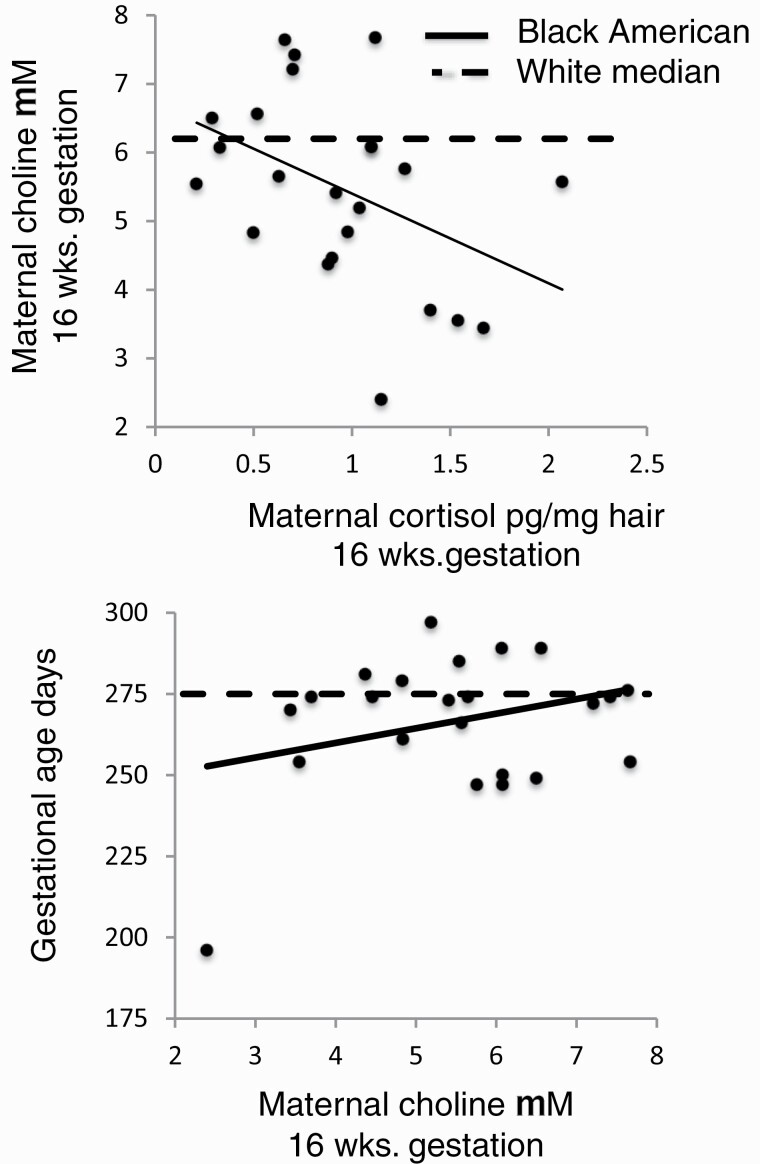
Black American mothers had higher 16-week cortisol hair concentrations associated with higher levels of depressive syndromes (table 1, supplementary table S5). Ancestry was the only significant main factor in an analysis of maternal choline concentration, considered with maternal cortisol concentration and presence of the biological father, Wald χ 2df1 = 5.96, P = .015 (supplementary table S6). Interaction for ancestry*biological father was Wald χ 2df1 = 8.10, P = .044 and for ancestry*maternal cortisol was Wald χ 2df1 = 4.64, P = .098. In regression analyses of maternal choline concentration for Black Americans, maternal cortisol was a significant negative factor β = −.477, P = .045 (figure 1), and father’s presence was negative but not significant, whereas for Whites, father was a significant positive factor β = .203, P = .048, and cortisol was positive but not significant.
Fig. 1.
Effects of maternal first-trimester hair cortisol concentration on maternal 16-wk choline plasma concentration (top) and effect of maternal 16-wk choline plasma concentration on gestational age at birth in Black Americans (bottom). Individual points and solid regression lines show values for Black Americans: for maternal first trimester hair cortisol and 16-wk choline μM, β = −.477, P = .045 (top); for maternal 16-wk choline μM and gestational age at birth, β = .335, P = .044 (bottom). Dashed lines in each plot are the median values of maternal choline (top) and gestational age at birth (bottom) for Whites.
Cortisol levels remained higher for Black American women than for Whites at 28 weeks gestation (table 1), but there was no relationship to choline level β = .061, P = .8.
Association of Lower Maternal Choline With Problems in Development
Choline and Differences in Gestational Age at Birth Between Black Americans and Whites
Gestational age at birth was lower for Black American neonates than for Whites. The difference for male neonates was 13 days, P = .03, and 6 days for female neonates, P = .08 (table 1). Seven of 23 Black Americans were born premature (<37 wk, 30%), compared to 13 of 126 Whites (10%), Fishers Exact Test P = .017.
An interaction between ancestry and choline concentration was associated with offspring gestational age at birth Wald χ 2df1 = 3.94, P = .047 (supplementary table S7). For Black Americans, maternal choline at 16 weeks gestation was positively associated with gestational age at birth, β = .335, P = .044 (figure 1). Use of prenatal vitamins with folate was also a significant positive factor in both groups. Choline at 28 weeks gestation did not affect gestational age at birth.
Five babies were born prior to 33 weeks gestation, 1 Black American, and 4 Whites. One woman had 2 previous preterm deliveries. The other 4 had previous term deliveries. Four had spontaneous onset of labor and one was induced; all had vaginal deliveries. Three took prenatal vitamins and 2 did not. Their mean choline at 16-weeks gestation was 4.61 μM (SD 1.42), compared to women with term deliveries, 7.42 μM (SD 2.01), P = .002.
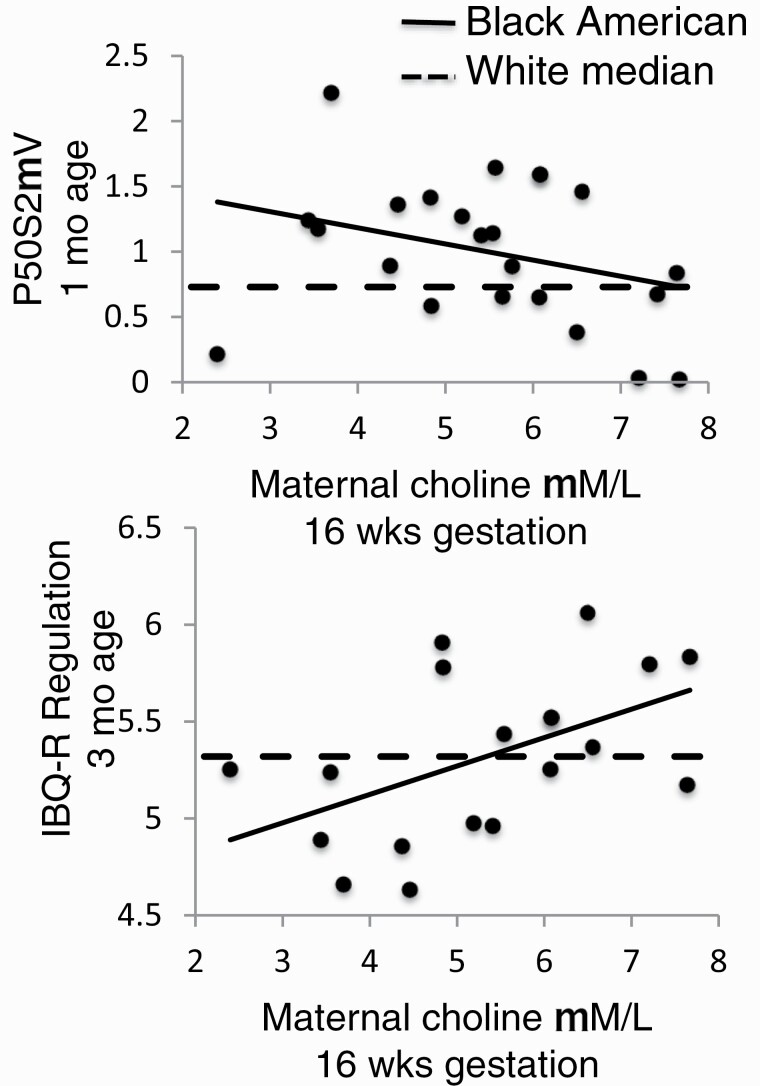
Black American Maternal Choline and Newborn P50S2μV
Larger P50 cerebral auditory evoked potential response to the second of paired stimuli, P50S2μV, indicates poorer development of inhibitory circuits in the hippocampus. Infants of Black American women had significantly greater P50S2μV, 0.99 μV (SD 0.54) than offspring of White women, 0.77 μV (SD 0.46), P = .041 (table 1). There was no difference in response amplitude to the first stimulus between the 2 groups. Maternal 16-week plasma choline and ancestral group had significant interaction for P50S2μV, Wald χ 2df1 = 4.16, P = .04 (supplementary table S8). Higher maternal choline was inversely associated with lower newborn P50S2μV in infants of Black American women, β = −.447, P = .021 (figure 2). Use of prenatal vitamins with folate by Black American women was also inversely associated with lower P50S2μV in the multivariate analysis, β = −.365, P = .049. Choline at 28 weeks gestation did not affect P50S2μV.
Fig. 2.
Effects of maternal 16-wk choline plasma concentrations on newborn P50S2μV at 1 mo of age (top) and infant IBQ-R Orienting/Regulation at 3 mo of age in Black Americans (bottom). Individual points and solid regression lines show values for Black Americans: for maternal 16-wk choline μM and newborn P50S2μV at 44 wk gestational age (nominally 1 mo of age), β = −.447, P = .021 (top); for maternal 16-wk choline μM and infant IBQ-R Orienting/Regulation at 52 wk gestational age (nominally 3 mo of age), β = .417, P = .050 (bottom). Dashed lines in each plot are the median values of P50S2μV (top) and IBQ-R Orienting/Regulation (bottom) for Whites.
Black American Maternal Choline, Infant Behavior Questionnaire-Revised (IBQ-R) Orienting/Regulation at 3 Months of Age, and Later Childhood Behavioral Problems
Higher maternal ratings of infants on IBQ-R Orienting/Regulation at 52 gestational weeks (nominally 3 months of age) indicate a child who is able to engage with new tasks, has longer duration of attention, and better soothing and cuddling with caregivers. Maternal 16-week choline concentration and maternal ancestral group had significant interaction for IBQ-R Orienting/Regulation, Wald χ 2df1 = 4.36, P = .037 (supplementary table S9). Black American maternal choline was positively associated with IBQ-R Orienting/Regulation, β = .417, P = .050 (figure 2). IBQ-R Orienting/Regulation was not associated with maternal postpartum CESD depression, STAI-S anxiety, PSS stress, or PSI parenting stress ratings (supplementary table S9).
A previously established risk factor in this cohort for decreased IBQ-R Orienting/Regulation is maternal infection in the first trimester.31 Plasma choline at 16 weeks gestation > 7μM was equally protective for offspring of Black American and White mothers (supplementary table S10). Choline 7 μM is associated with adequate choline intake and is 1 SD below the mean obtained with phosphatidylcholine supplementation in the trial described in the next section.28 However, the majority of both Black American N = 19 (83%) and of White women N = 82 (65%) had choline <7 μM.
IBQ-R Orienting/Regulation in Black American infants was inversely associated with newborn P50S2μV, r = −.502, P < .05. Eleven Black American children received periodic maternal ratings later in childhood on the Child Behavior Checklist 1½–5 years (CBCL 1½–5); the latest ratings were at 48 months of age for 9 children. For the CBCL 1½–5, higher scores indicate more problems. Their newborn P50S2μV was positively associated with more problems in Sleep r = .682, P < .05, Attention r = .605, P < .05, and Aggression r = .661, P < .05.
Effects of Perinatal Phosphatidylcholine Supplementation in Black Americans Who Participated in a Previous Randomized, Placebo-Controlled Clinical Trial
Fifteen Black American women enrolled in a previous trial of daily 6300 mg phosphatidylcholine supplementation beginning at 17 weeks gestation, with postnatal supplementation of the infants through the 52nd gestational week, approximately 3 months of age.28 Mothers received the equivalent of twice the US Food and Drug Administration recommended daily minimum amount of choline in addition to their dietary intake. Seven received phosphatidylcholine and 8 placebo.
Offspring of Black American mothers who received phosphatidylcholine had longer mean gestational age at birth 271.8 days (SE 5.2), compared to infants whose mothers received placebo 258.1 days (SE 4.9), Wald χ 2df1 = 3.62, P = .057 (supplementary table S11). Black American newborns whose mothers received phosphatidylcholine as well as themselves postnatally had lower mean P50S2 0.64μV (SE 0.25), compared to infants and mothers who received placebo 1.45μV (SE 0.28), Wald χ 2df1 = 3.79, P = .052.
Mothers rated their child’s behavior problems at 40 months of age on the CBCL-1½–5 years. Male children of Black American mothers had significantly lower mean percentile ratings of Withdrawn problems in social behavior if they and their mothers received phosphatidylcholine, 51.3 percentile (SE 4.9), than if they and their mothers received placebo, 68.2 percentile (SE 6.3), P = .034. Differences in female infants were not significant, Child sex*Phosphatidylcholine Wald χ 2df1 = 6.62, P = .010. A similar interaction was observed for Attention problems, phosphatidylcholine-treated mothers and male infants 51.6 percentile (SE 2.0), compared to placebo-treated mothers and male infants 65.4 percentile (SE 2.5), P < .001, with no significant difference in females, Child sex*Phosphatidylcholine Wald χ 2df1 = 4.72, P = .030.
Discussion
Black, African American women in this cohort had the lowest 16-week gestation plasma choline of any ancestry group. To what extent these women in a moderate-size urban area are typical of Black Americans in other settings is unknown. However, this small cohort had risk factors related to their lower choline that bear further investigation. Dietary surveys comparing Black Americans to Whites in the same urban setting report that both generally fail to reach recommended choline intake, with no significant difference between groups.51 In this cohort, obesity, generally considered a hallmark of poor diets, was less frequent in Black Americans than Whites and not a factor in choline concentration.52 Household income, which could affect diet, was not related to choline concentration. Even in the lowest economic quartile, Black Americans’ choline was significantly lower than Whites. Genotypes associated with lower choline, notably in PEMT, were not more frequent in Black Americans compared to Whites, and Black American mothers tended to have lower choline than Whites with the same genotype. Other aspects of African ancestry are not likely to be responsible for Black Americans’ lower choline, because women in Uganda have higher choline levels.
In monitored laboratory environments, dietary choline intake is not closely related to plasma choline.53 Black Americans, in particular, may have declining plasma choline even with adequate choline and folic acid intake.54 Other factors, such as maternal cortisol, may regulate plasma concentrations by determining the portioning of choline between maternal organs and the plasma and thereby to the fetus. Maternal stress, reflected biologically in increased cortisol in their first-trimester hair samples, was the factor most significantly associated with Black American choline concentration. Presence of the biological father in the household, which was associated with lower cortisol and higher choline concentrations in White women, did not have a similar buffering effect for Black American women. Stress and associated cortisol secretion have previously been found to suppress plasma choline in surgical patients.55 A more detailed consideration of stress in Black Americans and its mediating factors would be needed to establish how increased cortisol arises and affects plasma choline in early gestation. Black American women experience considerable high levels of systemic racism and discrimination that is a major cause of their stress.17,56
Low maternal choline levels in Black American women had significant association with problems in the development of the offspring, beginning with lower gestational age at birth. Choline supplementation reduces the expression of α7-nicotinic receptors and acetylcholinesterase in mouse placenta, together with other effects on placental inflammation and angiogensis.57,58 Placental cholinergic mechanisms appear to interact with the decrease in progesterone in the initiation of parturition, but how lower choline levels earlier in gestation adversely affect the development of placental cholinergic mechanisms that might then later fail to prevent preterm birth requires further investigation.59
Poorer development of inhibitory neurocircuits was assessed at 1 month by P50 auditory evoked potentials. Subsequent problems in child development were apparent at 3 months of age as lower IBQ-R Orienting/Regulation. The behavioral problems assessed by the IBQ-R and associated with choline concentration were notably not associated with other differences between Black Americans and Whites, including the presence of the biological father or the mother’s own adverse childhood experiences. Other prenatal insults, specifically maternal infections, that have adverse effects on early childhood development, were ameliorated by higher choline in both Black Americans and Whites. Thus, despite the significant social and symptomatic differences between Black Americans and Whites, higher choline plasma concentrations have similar beneficial effects in pregnancies of both groups. The salient difference is that few Black American women have adequate choline concentrations at 16 weeks gestation, when critical events in fetal brain development are occurring.
At 28 weeks, Black American women continued to have elevated hair cortisol, a biomarker of their stress associated with lower choline levels at 16 weeks gestation. At 28 weeks, their choline levels were no longer related to their cortisol levels and no longer decreased relative to Whites. Increased choline levels are generally observed during the course of pregnancy.47 One mechanism is the increasing estrogen stimulation of PEMT transcription, as estrogen levels rise during pregnancy.48 Dietary intake may also increase as morning sickness subsides. The mothers in this study had acknowledged their pregnancy and sought prenatal care, but they may not have realized the need to increase their nutrition in the first trimester as fetal brain and other organ development begins. For folic acid and Vitamins A and D, the fortification of processed foods with these nutrients is mandated in many countries, including the United States to protect early fetal development, even if the mother herself does not increase nutrient intake early in pregnancy. Choline is not currently fortified in foods, and therefore, this safety net is incomplete.
The developmental pathway to mental illnesses is multi-faceted. Other pathological factors in childhood, adolescence, and early adulthood have major effects as well. Stress after birth has been proposed to be a significant pathogenic factor in other under-represented groups and is certainly a pathogenic factor in Black Americans.60 Primary interventions both before and after birth are needed to prevent mental illness.61 Although prenatal risk alone may not determine who develops mental illnesses, many mentally ill patients have problems in cerebral P50 inhibition similar to those observed in newborns in this study and retrospectively had childhood problems in attention and social withdrawal that may have had prenatal origins.62 The present study, with limitations noted in the Supplement, finds that choline, like folic acid for spina bifida and facial clefts and Vitamin D for autistic traits, is a potentially useful prenatal preventive for these early developmental facets of mental illnesses that are difficult to treat after birth.63,64 Black American women in this study sought timely prenatal care and took prenatal vitamins. Despite the American Medical Association recommendation, no current prenatal vitamin contains even the US Food and Drug Administration minimum daily recommended amount of choline.21,23 This study suggests that for Black American children, in particular, the addition of prenatal maternal choline supplements may enhance their early development despite the challenges faced by their mothers.
Funding
National Institute of Child Health and Human Development (K12HD001271-11 to M.C.H.); National Center for Advancing Translational Sciences (UL1 TR001082); National Institute of Diabetes and Digestive and Kidney Diseases (R01DK56350 to S.Z.); The Institute for Children’s Mental Disorders; and The Anschutz Foundation.
Supplementary Material
Acknowledgments
The late Randal G. Ross designed and initiated this study. The late Carl Bell encouraged the National and American Medical Associations to advocate for increased choline in prenatal vitamins based on this study. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Bresnahan M, Begg MD, Brown A, et al. . Race and risk of schizophrenia in a US birth cohort: another example of health disparity? Int J Epidemiol. 2007;36(4):751–758. [DOI] [PubMed] [Google Scholar]
- 2.Paksarian D, Merikangas KR, Calkins ME, Gur RE. Racial-ethnic disparities in empirically-derived subtypes of subclinical psychosis among a U.S. sample of youths. Schizophr Res. 2016;170(1):205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zablotsky B, Alfordf JM. Racial and ethnic differences in the prevalence of attention-deficit/hyperactivity disorder and learning disabilities among U.S. children aged 3–17 years. Centers for Disease Control and Prevention. National Center for Health Statistics. NCHS Data Brief 2020;No. 358. https://www.cdc.gov/nchs/products/databriefs/db358.htm. Accessed June 1, 2020. [PubMed] [Google Scholar]
- 4.Nevison C, Zahorodny W. Race/ethnicity-resolved time trends in United States ASD prevalence estimates from IDEA and ADDM. J Autism Dev Disord. 2019;49(12):4721–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Susser ES, Lin SP. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944-1945. Arch Gen Psychiatry. 1992;49(12):983–988. [DOI] [PubMed] [Google Scholar]
- 6.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167(3):261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckhof, G. Fever and infections in pregnancy and risk of attention deficit/hyperactivity disorder in the offspring. J Child Psychol Psychiatry. 2016;57:540–548. [DOI] [PubMed] [Google Scholar]
- 8.Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry. 2009;195(1):7–14. doi: 10.1192/bjp.bp.108.051672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown AS, Meyer U. Maternal immune activation and neuropsychiatric illness: a translational research perspective. Am J Psychiatry. 2018;175(11):1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y, Lu YC, Jacobs M, et al. . Association of prenatal maternal psychological distress with fetal brain growth, metabolism, and cortical maturation. JAMA Netw Open. 2020;3(1):e1919940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20(3):441–451. [DOI] [PubMed] [Google Scholar]
- 12.Rich-Edwards JW, Kleinman K, Abrams A, et al. . Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J Epidemiol Community Health. 2006;60(3):221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urizar GG Jr, Yim IS, Kofman YB, et al. . Ethnic differences in stress-induced cortisol responses: increased risk for depression during pregnancy. Biol Psychol. 2019;147:107630. [DOI] [PubMed] [Google Scholar]
- 14.Almeida J, Bécares L, Erbetta K, Bettegowda VR, Ahluwalia IB. Racial/Ethnic inequities in low birth weight and preterm birth: the role of multiple forms of stress. Matern Child Health J. 2018;22(8):1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillespie SL, Christian LM, Alston AD, Salsberry PJ. Childhood stress and birth timing among African American women: cortisol as biological mediator. Psychoneuroendocrinology. 2017;84:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Kershaw T, Ettinger AS, Higgins C, Lu MC, Chao SM. Association between life event stressors and low birth weight in African American and white populations: findings from the 2007 and 2010 Los Angeles Mommy and Baby (LAMB) surveys. Matern Child Health J. 2015;19(10):2195–2205. [DOI] [PubMed] [Google Scholar]
- 17.Mendez DD, Hogan VK, Culhane JF. Institutional racism, neighborhood factors, stress, and preterm birth. Ethn Health. 2014;19(5):479–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giurgescu C, Slaughter-Acey JC, Templin TN, Misra DP. The impact of symptoms of depression and walking on gestational age at birth in African American Women. Womens Health Issues. 2017;27(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dueker G, Chen J, Cowling C, Haskin B. Early developmental outcomes predicted by gestational age from 35 to 41weeks. Early Hum Dev. 2016;103:85–90. [DOI] [PubMed] [Google Scholar]
- 20.Groth SW, Stewart PA, Ossip DJ, Block RC, Wixom N, Fernandez ID. Micronutrient intake is inadequate for a sample of pregnant African-American Women. J Acad Nutr Diet. 2017;117(4):589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Food and Drug Administration. Nutrition Labeling Requirements.https://www.fda.gov/media/99069/download. Accessed March 1, 2020.
- 22.Mills B, Dyer N, Pacheco D, Brinkley D, Owen MT, Caughy MO. Developmental transactions between self-regulation and academic achievement among low-income African American and Latino children. Child Dev. 2019;90(5):1614–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Medical Association. Proceedings of the 2017 Annual Meeting House of Delegates, https://www.ama-assn.org/about/proceedings-2017-annual-meetinghouse-delegates. Accessed November 27, 2017.
- 24.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18(4):1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu BT, Dyer RA, King DJ, Richardson KJ, Innis SM. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS One. 2012;7(8):e43448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boeke CE, Gillman MW, Hughes MD, Rifas-Shiman SL, Villamor E, Oken E. Choline intake during pregnancy and child cognition at age 7 years. Am J Epidemiol. 2013;177(12):1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross RG, Hunter SK, Hoffman MC, et al. . Perinatal phosphatidylcholine supplementation and early childhood behavior problems: evidence for CHRNA7 moderation. Am J Psychiatry. 2016;173(5):509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caudill MA, Strupp BJ, Muscalu L, Nevins JEH, Canfield RL. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: a randomized, double-blind, controlled feeding study. FASEB J. 2018;32(4):2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson SW, Carter RC, Molteno CD, et al. . Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: a randomized, double-blind, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2018;42(7):1327–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman R, Hunter SK, Law AJet al. . Higher gestational choline levels in maternal infection protect infant brain development. J Pediatrics. 2019;208:198–206.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman MC, Hunter SK, D’Alessandro A, Noonan K, Wyrwa A, Freedman R. Interaction of maternal choline levels and prenatal Marijuana’s effects on the offspring. Psychol Med. 2020;50(10):1716–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunter SK, Hoffman MC, D’Alessandro A, et al. . Male fetus susceptibility to maternal inflammation: c-reactive protein and brain development [published online ahead of print December 2019]. Psychol Med. doi: 10.1017/S0033291719003313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedman R, Hunter SK, Law AJ, et al. . Maternal choline and respiratory coronavirus effects on fetal brain development. J Psychiatr Res. 2020;128:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin LF, Hall MH, Ross RG, Zerbe G, Freedman R, Olincy A. Physiology of schizophrenia, bipolar disorder, and schizoaffective disorder. Am J Psychiatry. 2007;164(12):1900–1906. [DOI] [PubMed] [Google Scholar]
- 36.Olincy A, Blakeley-Smith A, Johnson L, Kem WR, Freedman R. Brief report: initial trial of alpha7-nicotinic receptor stimulation in two adult patients with autism spectrum disorder. J Autism Dev Disord. 2016;46(12):3812–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Micoulaud-Franchi JA, Vaillant F, Lopez R, et al. . Sensory gating in adult with attention-deficit/hyperactivity disorder: event-evoked potential and perceptual experience reports comparisons with schizophrenia. Biol Psychol. 2015;107:16–23. [DOI] [PubMed] [Google Scholar]
- 38.Kisley MA, Polk SD, Ross RG, Levisohn PM, Freedman R. Early postnatal development of sensory gating. Neuroreport. 2003;14(5):693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter SK, Kisley MA, McCarthy L, Freedman R, Ross RG. Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: parental psychosis, maternal depression, and nicotine use. Schizophr Bull. 2010;119:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith E, Crawford T, Thomas M, Reid V. Schizotypy and sensory gating: a 6-month-old EEG study. Schizophr Bull. 2018;44:S301–302. [Google Scholar]
- 41.Goff WR, Williamson PD, Vangilder JC, Allison T, Fisher TC. Neural origins of long latency evoked potentials recorded from the depth and from the cortical surface of the brain in man. Prog Clin Neurophysiol. 1990;7:126–145. [Google Scholar]
- 42.Miller CL, Freedman R. The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neuroscience. 1995;69(2):371–381. [DOI] [PubMed] [Google Scholar]
- 43.Bayatti N, Moss JA, Sun L, et al. . A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex. 2008;18(7):1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zecevic N, Hu F, Jakovcevski I. Cortical interneurons in the developing human neocortex. Dev Neurobiol. 2011;71:18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314(5805):1610–1613. [DOI] [PubMed] [Google Scholar]
- 46.Hyde TM, Lipska BK, Ali T, et al. . Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31(30):11088–11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orczyk-Pawilowicz M, Jawien E, Deja S, Hirnle L, Zabek A, Mlynarz P. Metabolomics of human amniotic fluid and maternal plasma during normal pregnancy. PLoS One. 2016;11(4):e0152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Resseguie M, Song J, Niculescu MD, da Costa KA, Randall TA, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007;21(10):2622–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ACOG (American College of Obstetricians and Gynecologists), Society for Maternal-Fetal Medicine. Guidance 700: Methods for estimating the due data.2017. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/methods-for-estimating-the-due-date. Accessed April 1, 2020.
- 50.Bosquet Enlow M, White MT, Hails K, Cabrera I, Wright RJ. The infant behavior questionnaire-revised: factor structure in a culturally and sociodemographically diverse sample in the united states. Infant Behav Dev. 2016;43:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brunst KJ, Wright RO, DiGioia K, et al. . Racial/ethnic and sociodemographic factors associated with micronutrient intakes and inadequacies among pregnant women in an urban US population. Public Health Nutr. 2014;17(9):1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siega-Riz AM, Bodnar LM, Savitz DA. What are pregnant women eating? Nutrient and food group differences by race. Am J Obstet Gynecol. 2002;186(3):480–486. [DOI] [PubMed] [Google Scholar]
- 53.Abratte CM, Wang W, Li R, Axume J, Moriarty DJ, Caudill MA. Choline status is not a reliable indicator of moderate changes in dietary choline consumption in premenopausal women. J Nutr Biochem. 2009;20(1):62–69. [DOI] [PubMed] [Google Scholar]
- 54.Hung J, Abratte CM, Wang W, Li R, Moriarty DJ, Caudill MA. Ethnicity and folate influence choline status in young women consuming controlled nutrient intakes. J Am Coll Nutr. 2008;27(2):253–259. [DOI] [PubMed] [Google Scholar]
- 55.Ozarda Ilçöl Y, Ozyurt G, Kilicturgay S, Uncu G, Ulus IH. The decline in serum choline concentration in humans during and after surgery is associated with the elevation of cortisol, adrenocorticotropic hormone, prolactin and beta-endorphin concentrations. Neurosci Lett. 2002;324(1):41–44. [DOI] [PubMed] [Google Scholar]
- 56.Chambers BD, Arabia SE, Arega HA, et al. . Exposures to structural racism and racial discrimination among pregnant and early postpartum Black women living in Oakland, California. Stress & Health. 2020;36:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu WL, Adams CE, Stevens KE, Chow KH, Freedman R, Patterson PH. The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain Behav Immun. 2015;46:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kwan STC, King JH, Yan J, et al. . Maternal choline supplementation during murine pregnancy modulates placental markers of inflammation, apoptosis and vascularization in a fetal sex-dependent manner. Placenta. 2017;53:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen YS, Brennecke SP, King RG. Human placental choline acetyltransferase activity at parturition. Placenta. 1992;13(3):255–264. [DOI] [PubMed] [Google Scholar]
- 60.McKenzie K, Jones P, Lewis S, et al. . Lower prevalence of pre-morbid neurological illness in African-Caribbean than White psychotic patients in England. Psychol Med. 2002;32(7):1285–1291. [DOI] [PubMed] [Google Scholar]
- 61.Arango C, Díaz-Caneja CM, McGorry PD, et al. . Preventive strategies for mental health. Lancet Psychiatry. 2018;5(7):591–604. [DOI] [PubMed] [Google Scholar]
- 62.Rossi A, Pollice R, Daneluzzo E, Marinangeli MG, Stratta P. Behavioral neurodevelopment abnormalities and schizophrenic disorder: a retrospective evaluation with the Childhood Behavior Checklist (CBCL). Schizophr Res. 2000;44(2):121–128. [DOI] [PubMed] [Google Scholar]
- 63.Wilcox AJ, Lie RT, Solvoll K, et al. . Folic acid supplements and risk of facial clefts: national population based case-control study. BMJ. 2007;334(7591):464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vinkhuyzen AAE, Eyles DW, Burne THJ, et al. . Gestational vitamin D deficiency and autism-related traits: the Generation R Study. Mol Psychiatry. 2018;23(2):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.