Abstract
Disturbed self-experience has been reported as a characteristic feature of schizophrenia since the first formulation of its diagnostic concept; however, only in the last 2 decades an explicit notion of basic Self-disturbance, or Self-Disorders (SD), has emerged as target for a systematic research program. We conducted systematic searches in bibliographical databases to identify cross-sectional studies that explored SD across different diagnostic groups and explored diagnostic ascription within or outside schizophrenia spectrum disorders (SSD) as main outcome. Data were pooled using fixed- and random-effects meta-analysis models. Heterogeneity was assessed using stratified meta-analyses and meta-regression. Of 218 identified studies, 32 were included in the systematic review and 27 in the meta-analysis. Patients diagnosed with SSD scored higher on measures of SD than healthy controls (HC) (Hedges’ g = 1.8; 95% CI = 1.5 to 2.0), individuals diagnosed with other mental illness (OMI) (1.9; 1.6 to 2.2), bipolar or affective disorders (1.8; 1.4 to 2.2), and clinical high risk for psychosis (CHR) (1.6; 0.9 to 2.4). Patients with schizotypy or schizotypal personality disorder scored higher on measures of SD than OMI (1.5; 1.3 to 1.8) and HC (1.4; 1.1 to 1.7). Patients with first-episode psychosis scored higher on measures of SD than HC (2.5; 2.1 to 2.9) and OMI (1.6; 1.2 to 2.1). Subjects at CHR scored higher on measures of SD than HC (2.0; 1.7 to 2.2) and OMI (19; 1.6 to 2.2). Overall, heterogeneity ranged from negligible to high, especially in comparisons of the target group with OMI, probably as a reflection of the immanent diagnostic heterogeneity of this group. The findings suggest that SD selectively aggregate within schizophrenia spectrum disorders as compared to other mental disorders and that they could be a central phenotypic marker of vulnerability to schizophrenia across the different shades of severity of its spectrum of disorders.
Keywords: schizophrenia spectrum, subjectivity, self-disorder, phenomenology, diagnosis
“I remember very well the day it happened. We were staying in the country and I had gone for a walk… at that instant a strange feeling came over me, a feeling hard to analyze…It was the first appearance of those elements which were always present in later sensations of unreality: illimitable vastness, brilliant light, and the gloss and smoothness of material things.”
Sechehaye, 1951
“The voices and so on were not that important. I think that the enduring and pervasive feeling of being unreal is the disease itself. When I realized this condition of looking at myself as in a movie was permanent, I understood it would eventually destroy the core of my life.”
Møller and Husby, 2000
“I am seven, or eight, […] looking out at the sunny day…And then something odd happens. My awareness (of myself, of him, of the room, of the physical reality around and beyond us) instantly grows fuzzy. Or wobbly. I think I am dissolving. I feel -my mind feels- like a sand castle with all the sand sliding away in the receding surf.”
Saks, 2007
“The clinical symptoms come and go, but this nothingness of the self is permanently there… By nothingness, I mean a sense of emptiness, a painful void of existence that only I can feel. My thoughts, my emotions, and my actions, none of them belong to me any more. This omnipotent and omnipresent emptiness has taken control of everything. I am an automaton, but nothing is working inside me.”
Kean, 2009
Introduction
These touching quotes, extrapolated from wider, first personal narratives1–3 or clinical cases,4 illuminate essential experiential features of vulnerability to schizophrenia spectrum disorders (SSD). That is, a profound transformation of subjectivity antedating the onset of major symptoms (ie, positive, negative, disorganized), yet conferring a pervasive and painfully prolonged coloring to the entire experiential field. A coloring which is accompanied by micro-experiences of self-alienation such as feelings of derealization, perplexity, depersonalization, reduced self-presence, and alteration of the stream of thought. This panoply of subtle, not-yet psychotic anomalies of subjective experiences have been recursively described in detail in classical European psychopathology5–9 (see comprehensive overviews10–14 and clinical exemplifications15) and ascribed as a fundamental descriptive aspect of schizophrenia since the inception of the concept.16 However, only in the last 2 decades, an explicit notion of basic Self-disturbance or Self-Disorders (hereafter SD), has emerged as target for a systematic research program, together with the development of an ad hoc assessment tool, such as the Examination of Anomalous Self-Experience (EASE).17
On a psychopathological level (basically as biographically exemplified in the exergo quotes1–3), SD are not contingent symptomatic constellations erratically enriching the clinical presentation, but rather express a fundamental and enduring (ie, trait-like) distortions of subjectivity. This might involve an unstable sense of self-presence and first-person perspective, fading sense of immersion in the surrounding world, a feeling of volatile self-identity and coherence, disturbances of the tacit fluidity of the stream of though, hyper-reflexivity, and perplexity (see EASE17 for a systematization).
Crucially, although expressed linguistically, these experiential anomalies, maintain a certain allure of ineffability as they emerge from a basic level of experience (so-called “minimal” or “core self” in contemporary cognitive science and phenomenology) implicitly conferring a sense of self-presence and self-familiarity to all subjective experiences.10 This first personal articulation of experience, which grounds its mine-ness and for-me-ness, is clinically discernible from the more sophisticated level of narrative, autobiographical self.15,18 The narrative self indeed coincides with the personal identity, evolving throughout life and heavily dependent on language, social interactions, and biography, and includes characterological, temperamental, and cognitive dispositions.
On a research level, SD are a promising candidate phenotype to anchor the validity of the extended SSD (ie, according to Meehl19,20 from a premorbid, so-called “schizotaxic,” predisposition to overt schizophrenia through intermediate schizotypal configurations of increasing clinical severity).19–22 Furthermore, SD typically emerge in late childhood and early adolescence and therefore offer a suitable developmental target phenotype antedating the development of major schizophrenia spectrum conditions.23–28
Overall, these features could be important both for translational purposes, such as early differential diagnosis of SSD vulnerability (eg, further improving current Clinical High-Risk stratification23,29–31 and enhancing the resolution power of family high-risk approach) and for etiopathogenetic research (eg, the genetic architecture and transgenerational vulnerability to schizophrenia22).
However, despite the literature seems to indicate a relative prevalence of SD within the SSD, the extent of such selective aggregation remains undefined. We therefore performed a meta-analysis to test the specificity of the association of SD with SSD diagnoses and quantify the magnitude of this association with respect to the different configurations within the SSD and outside of it.
Materials and Methods
Search Strategy
The study complies with the requirements of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)32 and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline.33
Data were retrieved with a search in PubMed/Medline and the Cochrane library (https://www.cochranelibrary.com/). The following inclusion criteria were applied:
interval from 2003 (year of the definition of the construct of SD (object of the current study), to August 31, 2020;
published in English;
published in peer-review journals, not merely in abstract;
clinical diagnosis made with a validated diagnostic procedure;
data on SD collected with a validated tool;
cross-sectional design;
original samples.
The following keywords were used: “Self-disorders” and “anomalous Self-experience” matched with “schizophrenia,” “schizophrenia spectrum disorders,” “schizotypy,” “psychosis,” “Ultra-high Risk,” “Clinical high risk,” “At Risk Mental States.”
A first reviewer inspected the title and abstract of the retrieved articles (M.P.), with a second reviewer cross-checking the first inspection to scan for potentially relevant studies (A.P.). The reviewers then inspected the full texts of the articles that were identified as relevant to check the content against inclusion criteria. A third reviewer (A.R.) contributed to solving discrepancies until consensus was reached. The same procedure was applied to the references that were extracted by the articles and related reviews on the topic.14,34 Details on the step-by-step extraction are in the PRISMA Flowchart (figure 1). Selected studies were evaluated for quality ratings (supplementary table S4). Quality of the studies was rated with the Wells et al’s Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). The NOS explores structural methodological aspects of observational studies such as the selection of the study groups, their comparability and the ascertainment of the exposure interest, conferring a star-based rating for each quality item. Studies were then rated as “poor,” “fair,” or “good” depending on their agreement with the expected requirements for a high-quality observational study.
Fig. 1.
Prisma flow diagram of study selection and inclusion.
Systematic Review
All studies that were deemed congruent with the aims and the criteria of the systematic review are reported in the supplementary table S1. Data extraction was done by one investigator (M.P.) and crosschecked by a second investigator (A.P.). Discrepancies were resolved through consensus meetings with expert investigators (A.R., J.P.).
We reported data about the first author of the study, the year of publication, the location of the study, the criteria and the instruments for clinical diagnosis, the tool that was used to measure SD, population of the study with details on diagnosis, sample size, gender ratio, mean age, results of SD assessment (mean scores), and key statistical findings derived from comparison of samples on SD measures.
Meta-analysis
Meta-analysis was applied to all studies with at least 2 groups that were compared with a measure of SD. Effect size was calculated as Hedges’ adjusted g with 95% Confidence Interval (CI). The calculated Hedges’ g and its variance were analyzed with the “metafor” package35 and the “meta” package36 running in R version 3.5.1.37 The results of both the fixed-effects and random-effects models were reported for pairwise meta-analyses of a target group and all potential analyzable comparison groups. Fixed-effects models aim to make a conditional inference only on the studies included in the meta-analysis.35 The random-effects models aim to provide inference about the average effect in the entire population from which the studies are expected to be drawn. In the interpretation of the results, we gave preference to the fixed-effects model, since the fixed-effects model does not inflate the role of small studies as the random-effects model does,38 and the fixed-effects model has greater power compared to the random-effects model.39 These aspects are relevant in estimating differences in a small number of studies with small sample sizes. When heterogeneity is null or reasonably small (ie, <30%, see below), the fixed-effects model is the best description of the common effect in the analyzed samples and discrepancy with random-effects model can be attributed to the inflation of the role of small studies that is typical of the random-effects model.40,41
We estimated heterogeneity among studies using the empirical Bayes estimator.42 Under the random-effects model, the Hartung and Knapp correction was applied.43 Heterogeneity was assessed with Cochran’s Q and I2 statistics.44 For I2, conventional thresholds were used: 30%–60% may represent moderate heterogeneity; 50%–90%: may represent substantial heterogeneity; 75%–100% represent considerable heterogeneity.45 The 95% CI for I2 were calculated on the empirical Bayes estimator and the Q-profile method.46 Egger’s test was not performed due to the inclusion of fewer than 10 studies. The radial plot was used to check for the adequacy of the models and outlier detection.47 Studies with estimates that were beyond 2 SDs at P < .01 from the common estimates were assumed to have a poor fit with the model (ie, they were flagged as potential outlier). When outliers were identified, the model was recalculated without the outliers.
Results
Overall, the search strategy identified 32 studies with independent samples that reported data on SD21,23,29–31,48–75 (supplementary table S1). There were 21 studies from European countries, of whom 12 from Scandinavian countries, 4 studies from Australia, 3 studies from Israel, 2 studies from the United States, and 1 from Korea. We noted the total lack of studies from Africa and South America.
The studies used a variety of tools to measure the presence of SD; earliest studies21,48–50 used SD-related scores derived from the Bonn Scale for the Assessment of Basic Symptoms (BSABS),76 an instrument for the assessment of Basic Symptoms (ie, the earliest, subjectively experienced putative prodromal manifestations of a psychotic risk); thereafter, subsequent studies used the Examination of Anomalous Self-Experience (EASE),17 a specifically developed, phenomenologically-inspired interview to explore an extensive array of disturbed self-experience. In addition to semi-structured clinical interviews (BSABS and EASE), the MMPI derived Self‐Disorder Scale (SDO)75 and the self-report questionnaire Inventory of Psychotic-like Anomalous Self-Experiences (IPASE)71 were developed and adopted in studies included in the current meta-analysis (see supplementary table S2 for a synthetic overview of their characteristics).
SD were assessed in a variety of clinical conditions, with the majority related to SSD (supplementary table S3). There were 14 studies reporting data on patients diagnosed with schizophrenia or a related disorder; there were 5 studies with data on patients diagnosed with schizotypal personality disorder, while 2 additional studies identified people with schizotypy on the bases of the responses on self-report measures of schizotypy such as the Schizotypal Personality Questionnaire77 and the Chapman scales on magical ideation, perceptual aberration, and social anhedonia.78 There were 7 studies with data on patients with first-episode of psychosis (FEP) and 11 studies with data on clinical high-risk (CHR) samples, selected with validated clinical interviews as the Structured Interview for Prodromal Syndromes (SIPS)79 and the Comprehensive Assessment of At Risk Mental States (CAARMS).80 Finally, there were 3 studies reporting data on patients diagnosed with bipolar or affective disorders, 2 studies reporting data on patients diagnosed with obsessive compulsive disorder, 1 study reporting data on borderline personality disorder, and 1 study reporting data on Asperger syndrome/Autistic Spectrum Disorder. Moreover, 9 studies reported data on patients diagnosed with other mental disorders, 2 studies reported data on subject with no mental illness, and 19 studies reported data on healthy controls (HC).
Criteria for diagnosis varied from DSM-III to DSM 5 and ICD 10, and the procedure for diagnosis included the use of a standardized interview as SCID in 14 studies, SIPS in 6 studies, and the CAARMS in 3 studies.
Quality was “poor” in 10 studies, “fair” in 12 studies, “good” in 10 studies. The most recent studies (2019–2020) were not more likely to be rated as “good” than older studies (χ 2 = 1.90; df = 2; P = .387).
Systematic Review: Main Findings
These studies showed globally a gradient in SD scores, with higher scores in SSD than in clinical conditions outside SSD and in healthy controls; moreover, within the psychotic spectrum, subjects with FEP present higher scores in comparison with CHR subjects, who present higher scores in comparison with HC (details in supplementary table S1).
Meta-analysis of the Studies With Comparison Groups
There were 27 studies that reported estimates of SD in 2 or more groups. The following target groups were evaluated: schizophrenia; schizotypy; FEP; CHR. The comparison groups can be seen in the resulting forest plot.
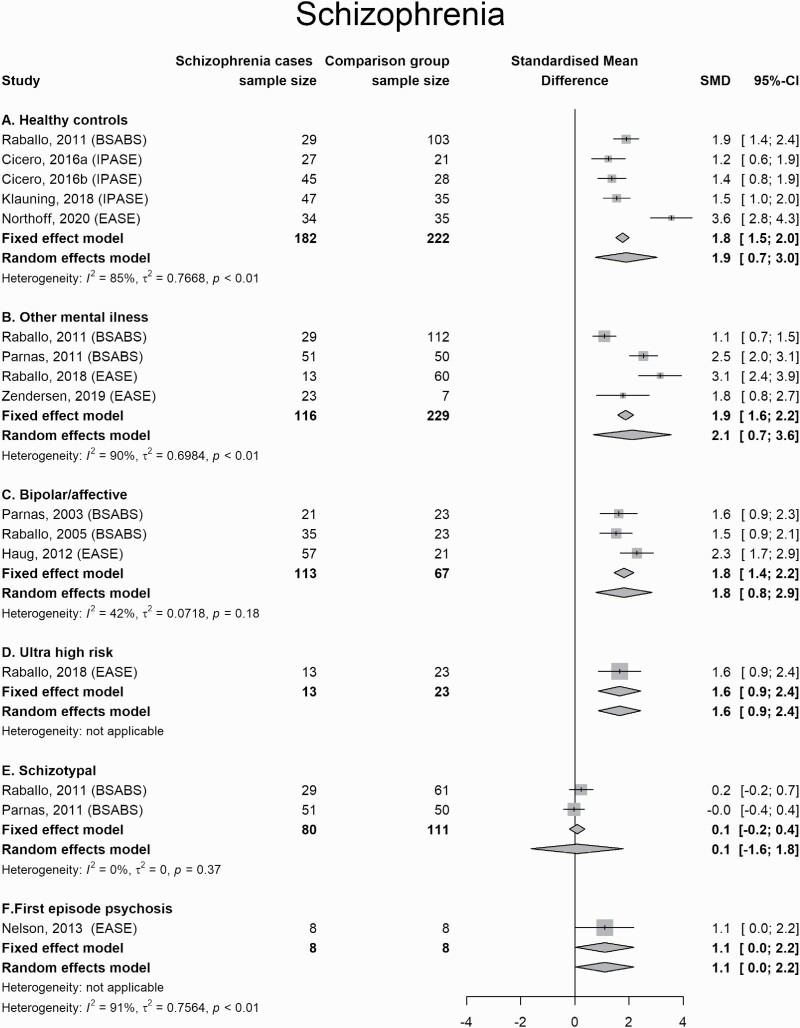
Patients diagnosed with schizophrenia and related disorders scored higher on measures of SD than healthy controls (Hedges’ g = 1.8; 95% CI = 1.5 to 2.0), individuals diagnosed with other mental illness (1.9; 1.6 to 2.2), patients diagnosed with bipolar or affective disorders (1.8; 1.4 to 2.2), and subjects deemed to be at clinical high risk for psychosis (1.6; 0.9 to 2.4). They did not differ from people identified with schizotypy or schizotypal personality disorder (0.1; −0.2 to 0.4) or those diagnosed with first-episode psychosis (1.1; 0.0 to 2.2) (figure 2).
Fig. 2.
Forest Plot for comparisons between schizophrenia and other conditions.
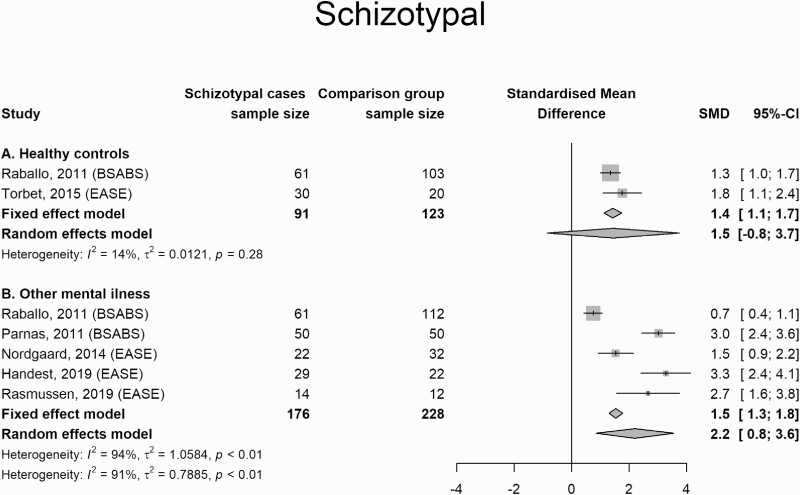
People who were diagnostically allocated in the area of schizotypy or schizotypal personality disorder scored higher on measures of SD than people with other mental illness (1.5; 1.3 to 1.8) and healthy controls (1.4; 1.1 to 1.7). For healthy controls, the random effect model didn’t reach the statistically significant threshold, but with I2 = 14% this might be attributed to inflation of small sample studies (figure 3).
Fig. 3.
Forest Plot for comparisons between schizotypy/schizotypal personality disorder and other conditions.
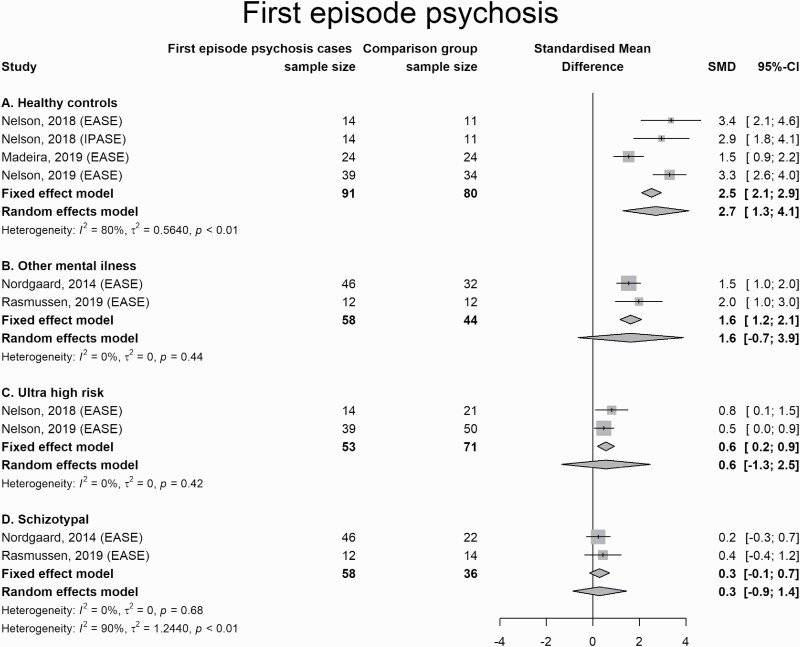
Patients with FEP scored higher on measures of SD than healthy controls (2.5; 2.1 to 2.9), scored higher than patients with other mental illness (1.6; 1.2 to 2.1; the random-effects model was not statistically significant but I2 was 0%), scored modestly higher than CHR subjects (0.6; 0.2 to 0.9), but not at the random-effects model (yet again with I2 = 0%), and were indistinguishable from those who were identified with schizotypy or schizotypal personality disorder (figure 4).
Fig. 4.
Forest Plot for comparisons between first-episode psychosis and other conditions.
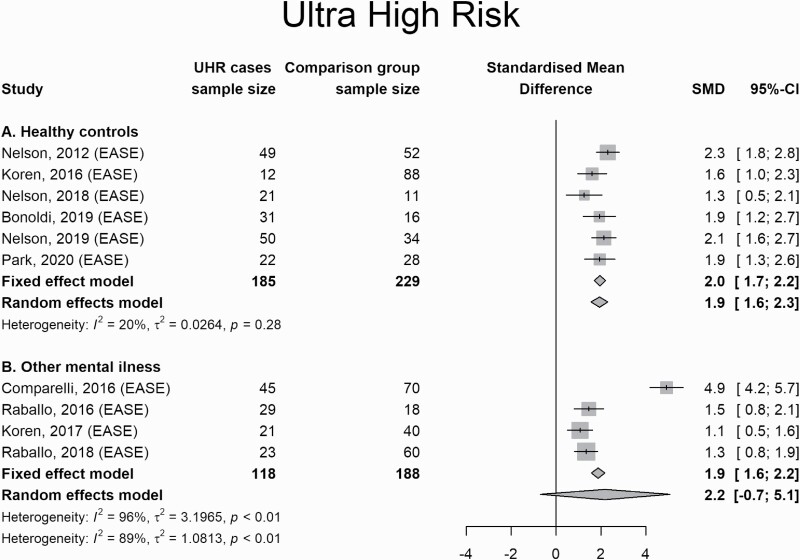
CHR subjects scored higher on measures of SD than healthy controls (2.0; 1.7 to 2.2) and those with other mental illness (1.9; 1.6 to 2.2), but not at the random-effects model, and in this case, I2 was considerable (96%), raising some doubt about the result of the fixed-effect model (figure 5).
Fig. 5.
Forest Plot for comparison between subjects at Ultra-High Risk for psychosis and other conditions.
Indeed, in this set of studies, one study58 is an outlier compared to the other studies’ effect sizes (supplementary figure S1). When this study is removed, CHR subjects were found to score higher than those with other mental illness with I2 = 0% (supplementary figure S1).
Overall, heterogeneity ranged from negligible to high, especially in comparisons of the target group with patients with other mental illness, probably as a reflection of the heterogeneity of the diagnosis in the comparison group (ie, Other mental illness includes by definition a substantial variety of diagnostic conditions not included in the other classes).
Discussion
To our knowledge, this is the first comprehensive meta-analysis addressing the differential risk of diagnostic ascription within or outside the schizophrenia spectrum in individuals experiencing SD. We found strong evidence for the validity of SD as a phenotypic marker of vulnerability to schizophrenia across the different shades of severity of its spectrum of disorders. Crucially, the level of SD is not just running in parallel with the degree of full-blown psychosis, since allegedly non-eminently psychotic components of the SSD such as schizotypal conditions exhibited commensurable levels of SD to schizophrenia (ie, the psychotic extreme of the SSD).
Strictly speaking, SD are not just another symptom domain which occurs in parallel with other symptoms clusters (see network analyses81,82 and qualitative narratives for a more in depth clinical grasp1–4) but rather enduring indicators of altered structures of experience. Therefore, both the chronological and psychopathological relation to symptoms is more likely a generative one, with SD reflecting necessary conditions for certain symptoms (eg, delusions, hallucinations, social withdrawal as well as schizotypal behavioral features) to emerge.83–86
The findings of the current MA confirm that SD intercept a specific schizophrenia spectrum-proneness which is distinguished from a broader vulnerability to psychosis and can inform future decisions with respect to the inclusion of SD among the descriptive criteria for SSD diagnosis or a refinement of the “distortions of self-experience” currently incorporated in the pertinent section of ICD-11.
Implications for Research and Clinical Praxis
The study confirms that the concept of SDs intercepts a valuable, quantitative phenotype for indexing the liability to SSD, including a variety of sub-psychotic configurations. This has important implications from the viewpoint of the construct validity of SSD, since SD could be conceptualized (in line with the original bleulerian description of “schizophrenias,” and the following spectrum declinations proposed for example by Meehl19,20 and Kety87) as a core vulnerability feature informing a continuum of expressivity ranging from subtle personality deviations to full-fledged clinical configurations. For this reason, the notion of SD, could be a tangible advancement for a more precise diagnostic identification of SSD, strengthening its clinical and research utility. Indeed, although constantly revised and debated, psychiatric classification continues to depend largely on clinical description. With respect to the SSD, current DSM 5 and ICD-10 polythetic-operational definitions highlight major symptom clusters but lack an organizing prototype-directed or a gestaltic intelligibility principle (ie, a recognizable pattern of coherence that unifies the diverse phenomenal features on the basis of reciprocal part-whole interrelations). Therefore, for example, the demarcation of schizophrenia from psychotic forms of affective illness, as well as of prodromal phases of schizophrenia from more benign at risk mental states remains difficult and often tardive.
In this respect, the results of the current study strengthen the rationale for exploring SD in those help-seekers who screened positive for CHR states. SD could in fact enrich CHR stratification, which focuses by definition on the imminent risk of transition to psychosis, with a further, prognostically relevant indication, ie, the SSD-proneness.
This should be better contextualized in light of some specific clinical features of SD. First, since SD typically emerge in late childhood and early adolescence, they provide an early, target phenotype for the identification of SSD proclivity in developmental years.23,24,88–90 Second, while contemporary early identification approaches (ie, ultra high-risk and basic symptoms criteria91) are explicitly designed to target the broader area of psychosis (which includes affective psychosis as well), SD selectively map SSD vulnerability. Third, since SD are enduring or trait-like experiences, endowed with a substantial temporal stability,81,82 as opposed to subclinical positive symptoms (eg, attenuated psychotic symptoms and/or so-called psychotic-like experiences), they allow a longer term prognostic vantage point compared to the time-window of the “imminent risk” of transition to psychosis.
Strengths and Limitations
Heterogeneity ranged from negligible to high, especially in comparisons of the target group with patients with other mental illness, probably as a reflection of the heterogeneity of the diagnosis in the comparison group. In most comparisons, the number of included studies was limited (often less than 5) and the sample size per study was rather modest. This is presumably an immanent consequence of the high level of clinical training, time and overall psychopathological competence required for the assessment of SD in its gold standard way (ie, via semi-structured interviews such as the EASE). Indeed, the EASE (and to a certain extent the BSABS) is a resource-intensive instrument (both in terms of administration length and continuity of training required), which provides an interviewer-based framework for the in-depth exploration of a clinically complex phenomenon such as basic SD (see refs.1–4,11,12,15). On the contrary, self-rating measures such as IPASE (which are faster to administer and do not presuppose training or supervision), might be less precise in the characterization of SD construct.61,85 Finally, the quality of the studies varied from poor to good, and this might have further impacted on heterogeneity. Indeed, in one sensitivity analysis, the elimination of a study that was identified as outlier according to validated statistical procedures (supplementary figure S1) resulted in clearer estimates and abatement of heterogeneity (supplementary figure S2).
Conclusion
In summary, there is meta-analytical evidence for the specific aggregation of SD in schizophrenia-spectrum disorders (ie, schizophrenia and schizotypal conditions) as compared to other mental illnesses and healthy controls. This offers key avenues for a more comprehensive characterization of core SSD phenotypes with potential implications for nosology, timely differential diagnosis, and etiological research.
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
The authors received no specific funding for this work.
References
- 1. Sechehaye M. Autobiography of a Schizophrenic Girl. New York, NY: Grine & Stratton; 1951. [Google Scholar]
- 2. Saks E. The Center Cannot Hold. London, UK: Virago Press; 2007. [Google Scholar]
- 3. Kean C. Silencing the self: schizophrenia as a self-disturbance. Schizophr Bull. 2009;35(6):1034–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Møller P, Husby R. The initial prodrome in schizophrenia: searching for naturalistic core dimensions of experience and behavior. Schizophr Bull. 2000;26(1):217–232. [DOI] [PubMed] [Google Scholar]
- 5. Berze J. Die primäre Insuffizienz der psychischen Aktivität: Ihr Wesen, ihre Erscheinungen und ihre Bedeutung als Grundstörungen der Dementia Praecox und der hypophrenen überhaupt. Leipzig, Germany: Frank Deuticke; 1914. [Google Scholar]
- 6. Gruhle H. Schizophrene Grundstimmung (Ich-Störrung). Psychologie der Schizophrenia. Berlin, Germany: Springer Verlag; 1929: 86–94. [Google Scholar]
- 7. Jaspers K. General Psychopathology (tr. Hoenig, J & Hamilton, M). London, UK: The John Hopkins University Press; 1959/1963. [Google Scholar]
- 8. Minkowski E. La schizophrénie. Psychopathologie des schizoïdes et des schizophrenes. Paris, France: Payot; 1927 [Google Scholar]
- 9. Tatossian A. La phénoménologie des psychoses. Paris, France: Masson; 1978. [Google Scholar]
- 10. Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophr Bull. 2003;29(3):427–444. [DOI] [PubMed] [Google Scholar]
- 11. Parnas J, Handest P. Phenomenology of anomalous self-experience in early schizophrenia. Compr Psychiatry. 2003;44(2):121–134. [DOI] [PubMed] [Google Scholar]
- 12. Parnas J, Jansson L, Sass L, Handest P. Self-experience in the prodromal phases of schizophrenia: a pilot study of first admissions. Neurol Psychiatry Brain Res. 1998;6:97–106. [Google Scholar]
- 13. Henriksen MG, Nordgaard J. Schizophrenia as a disorder of the self. J Psychopathol. 2014;20:435–441. [Google Scholar]
- 14. Parnas J, Henriksen MG. Disordered self in the schizophrenia spectrum: a clinical and research perspective. Harv Rev Psychiatry. 2014;22(5):251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raballo A. Self-disorders and the experiential core of schizophrenia spectrum vulnerability. Psychiatr Danub. 2012;24(Suppl 3):S303–S310. [PubMed] [Google Scholar]
- 16. Jansson L, Parnas J. The schizophrenic basic mood (self-disorder)’, by Hans W Gruhle (1929). Hist Psychiatry. 2020;31(3):364–375. [DOI] [PubMed] [Google Scholar]
- 17. Parnas J, Møller P, Kircher T, et al. . EASE: Examination of Anomalous Self-Experience. Psychopathology. 2005;38(5):236–258. [DOI] [PubMed] [Google Scholar]
- 18. Zandersen M, Parnas J. Identity disturbance, feelings of emptiness and the boundaries of the schizophrenia spectrum. Schizophr. Bull. 2019;45(1):106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meehl PE. Schizotaxia, schizotipy, schizophrenia. Am Psychol. 1962;17(12):827–838. [Google Scholar]
- 20. Meehl PE. Schizotaxia revisited. Arch Gen Psychiatry. 1989;46(10):935–944. [DOI] [PubMed] [Google Scholar]
- 21. Raballo A, Sæbye D, Parnas J. Looking at the schizophrenia spectrum through the prism of self-disorders: an empirical study. Schizophr Bull. 2011;37(2):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raballo A, Parnas J. The silent side of the spectrum: schizophrenia and the schizotaxic self. Schizophr Bull. 2011;37(5):1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raballo A, Monducci E, Ferrara M, et al. . Developmental vulnerability to psychosis: selective aggregation of basic self-disturbance in early onset schizophrenia. Schizophr Res. 2018;201:367–372. [DOI] [PubMed] [Google Scholar]
- 24. Koren D, Tzivoni Y, Schalit L, et al. . Basic self-disorders in adolescence predict schizophrenia spectrum disorders in young adulthood: A 7-year follow-up study among non-psychotic help-seeking adolescents. Schizophr Res. 2020;216:97–103. [DOI] [PubMed] [Google Scholar]
- 25. Brent BK, Seidman LJ, Thermenos HW, Holt DJ, Keshavan MS.. Self disturbances as a possible premorbid indicator of schizophrenia risk: a neurodevelopmental perspective. Schizophr. Res. 2014;152:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poletti M, Gebhardt E, Raballo A. Corollary discharge, self-agency, and the neurodevelopment of the psychotic mind. JAMA Psychiatry. 2017;74(11):1169–1170. [DOI] [PubMed] [Google Scholar]
- 27. Poletti M, Tortorella A, Raballo A. Impaired corollary discharge in psychosis and at-risk states: integrating neurodevelopmental, phenomenological, and clinical perspectives. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(9):832–841. [DOI] [PubMed] [Google Scholar]
- 28. Poletti M, Raballo A. uncanny mirroring: a developmental perspective on the neurocognitive origins of self-disorders in schizophrenia. Psychopathology. 2019;52(5):316–325. [DOI] [PubMed] [Google Scholar]
- 29. Nelson B, Thompson A, Yung AR. Basic self-disturbance predicts psychosis onset in the ultra high risk for psychosis “prodromal” population. Schizophr Bull. 2012;38(6):1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raballo A, Pappagallo E, Dell’ Erba A, et al. . Self-disorders and clinical high risk for psychosis: an empirical study in help-seeking youth attending community mental health facilities. Schizophr Bull. 2016;42(4):926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Værnes TG, Røssberg JI, Møller P. Anomalous self-experiences are strongly associated with negative symptoms in a clinical high-risk for psychosis sample. Compr Psychiatry. 2019;93:65–72. [DOI] [PubMed] [Google Scholar]
- 32. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stroup DF, Berlin JA, Morton SC, et al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 34. Hur JW, Kwon JS, Lee TY, Park S. The crisis of minimal self-awareness in schizophrenia: a meta-analytic review. Schizophr Res. 2014;152(1):58–64. [DOI] [PubMed] [Google Scholar]
- 35. Viechtbauer W. Conducting meta-analyses in R with the metaphor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 36. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, http://www.Rproject.org/, 2018. [Google Scholar]
- 38. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. [DOI] [PubMed] [Google Scholar]
- 39. Jackson D, Turner R. Power analysis for random-effects meta-analysis. Res Synth Methods. 2017;8(3):290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hedges L, Vevea J. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3(4):486–504. [Google Scholar]
- 41. Rice K, Higgins J, Lumley T. A re-evaluation of fixed effect (s) meta-analysis. J R Stat Soc Ser A Stat Soc. 2018;181:205–227. [Google Scholar]
- 42. Morris CN. Parametric empirical Bayes inference: Theory and applications (with discussion). J Am Stat Assoc. 1983;78(381):47–65. [Google Scholar]
- 43. Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693–2710. [DOI] [PubMed] [Google Scholar]
- 44. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206. [DOI] [PubMed] [Google Scholar]
- 45. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Viechtbauer W. Confidence intervals for the amount of heterogeneity in meta-analysis. Stat Med. 2007;26(1):37–52. [DOI] [PubMed] [Google Scholar]
- 47. Galbraith R. Some applications of radial plots. J Am Stat Assoc. 1994; 89: 1232–1242. [Google Scholar]
- 48. Parnas J, Handest P, Saebye D, Jansson L. Anomalies of subjective experience in schizophrenia and psychotic bipolar illness. Acta Psychiatr Scand. 2003;108(2):126–133. [DOI] [PubMed] [Google Scholar]
- 49. Raballo A, Maggini C. Experiential anomalies and self-centrality in schizophrenia. Psychopathology. 2005;38(3):124–132. [DOI] [PubMed] [Google Scholar]
- 50. Parnas J, Raballo A, Handest P, Jansson L, Vollmer-Larsen A, Saebye D. Self-experience in the early phases of schizophrenia: 5-year follow-up of the Copenhagen Prodromal Study. World Psychiatry. 2011;10(3):200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haug E, Lien L, Raballo A, et al. . Selective aggregation of self-disorders in first-treatment DSM-IV schizophrenia spectrum disorders. J Nerv Ment Dis. 2012;200(7):632–636. [DOI] [PubMed] [Google Scholar]
- 52. Koren D, Reznik N, Adres M, et al. . Disturbances of basic self and prodromal symptoms among non-psychotic help-seeking adolescents. Psychol Med. 2013;43(7):1365–1376. [DOI] [PubMed] [Google Scholar]
- 53. Nelson B, Thompson A, Yung AR. Not all first-episode psychosis is the same: preliminary evidence of greater basic self-disturbance in schizophrenia spectrum cases. Early Interv Psychiatry. 2013;7(2):200–204. [DOI] [PubMed] [Google Scholar]
- 54. Nordgaard J, Parnas J. Self-disorders and the schizophrenia spectrum: a study of 100 first hospital admissions. Schizophr Bull. 2014;40(6):1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Torbet G, Schulze D, Fiedler A, Reuter B. Assessment of self-disorders in a non-clinical population: Reliability and association with schizotypy. Psychiatry Res. 2015;228(3):857–865. [DOI] [PubMed] [Google Scholar]
- 56. Haug E, Øie M, Andreassen OA, et al. . Anomalous self-experience and childhood trauma in first-episode schizophrenia. Compr Psychiatry. 2015;56:35–41. [DOI] [PubMed] [Google Scholar]
- 57. Koren D, Lacoua L, Rothschild-Yakar L, Parnas J. Disturbances of the basic self and prodromal symptoms among young adolescents from the community: a pilot population-based study. Schizophr Bull. 2016;42(5):1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Comparelli A, Corigliano V, De Carolis A, et al. . Anomalous self-experiences and their relationship with symptoms, neuro-cognition, and functioning in at-risk adolescents and young adults. Compr Psychiatry. 2016;65:44–49. [DOI] [PubMed] [Google Scholar]
- 59. Koren D, Scheyer R, Reznik N, et al. . Basic self-disturbance, neurocognition and metacognition: A pilot study among help-seeking adolescents with and without attenuated psychosis syndrome. Early Interv Psychiatry. 2019;13(3):434–442. [DOI] [PubMed] [Google Scholar]
- 60. Martin B, Franck N, Cermolacce M, et al. . Fragile temporal prediction in patients with schizophrenia is related to minimal self disorders. Sci Rep. 2017;7(1):8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nelson B, Li E, Cicero DC, et al. . The construct validity of the Inventory of Psychotic-Like Anomalous Self-Experiences (IPASE) as a measure of minimal self-disturbance: preliminary data. Early Interv Psychiatry. 2019;13(3):686–691. [DOI] [PubMed] [Google Scholar]
- 62. Zandersen M, Parnas J. Borderline personality disorder or a disorder within the schizophrenia spectrum? A psychopathological study. World Psychiatry. 2019;18(1):109–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nilsson M, Arnfred S, Carlsson J, et al. . Self-Disorders in Asperger Syndrome Compared to Schizotypal Disorder: A Clinical Study. Schizophr Bull. 2020;46(1):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bonoldi I, Allen P, Madeira Let al. . Basic Self-Disturbances related to reduced anterior cingulate volume in subjects at Ultra-High Risk for psychosis. Front Psychiatry. 2019;10:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rasmussen AR, Nordgaard J, Parnas J. Schizophrenia-spectrum psychopathology in obsessive-compulsive disorder: an empirical study. Eur Arch Psychiatry Clin Neurosci. 2020;270(8):993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Madeira L, Pienkos E, Filipe T, et al. . Self and world experience in non-affective first episode of psychosis. Schizophr Res. 2019;211:69–78. [DOI] [PubMed] [Google Scholar]
- 67. Nelson B, Lavoie S, Gawęda Ł, et al. . The neurophenomenology of early psychosis: an integrative empirical study. Conscious Cogn. 2020;77:102845. [DOI] [PubMed] [Google Scholar]
- 68. Nordgaard J, Henriksen MG, Berge J, Nilsson LS. First-rank symptoms and self-disorders in schizophrenia. Schizophr Res. 2019;210:306–307. [DOI] [PubMed] [Google Scholar]
- 69. Park HY, Park K, Seo E, et al. . Reduced activation of the ventromedial prefrontal cortex during self-referential processing in individuals at ultra-high risk for psychosis. Aust N Z J Psychiatry. 2020;54(5):528–538. [DOI] [PubMed] [Google Scholar]
- 70. Northoff G, Sandsten KE, Nordgaard J, Kjaer TW, Parnas J. The self and its prolonged intrinsic timescale in schizophrenia [published online ahead of print July 2, 2020]. Schizophr Bull. 2021;47(1):170–179. doi: 10.1093/schbul/sbaa083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cicero DC, Neis AM, Klaunig MJ, Trask CL. The Inventory of Psychotic-Like Anomalous Self-Experiences (IPASE): development and validation. Psychol Assess. 2017;29(1):13–25. [DOI] [PubMed] [Google Scholar]
- 72. Cicero DC, Klaunig MJ, Trask CL, Neis AM. Anomalous self-experiences and positive symptoms are independently associated with emotion processing deficits in schizophrenia. Schizophr Res. 2016;176(2-3):456–461. [DOI] [PubMed] [Google Scholar]
- 73. Klaunig MJ, Trask CL, Neis AM, et al. . Associations among domains of self-disturbance in schizophrenia. Psychiatry Res. 2018;267:187–194. [DOI] [PubMed] [Google Scholar]
- 74. Gawęda Ł, Pionke R, Krężołek M, et al. . Self-disturbances, cognitive biases and insecure attachment as mechanisms of the relationship between traumatic life events and psychotic-like experiences in non-clinical adults - A path analysis. Psychiatry Res. 2018;259:571–578. [DOI] [PubMed] [Google Scholar]
- 75. Parnas J, Carter J, Nordgaard J. Premorbid self-disorders and lifetime diagnosis in the schizophrenia spectrum: a prospective high-risk study. Early Interv Psychiatry. 2016;10(1):45–53. [DOI] [PubMed] [Google Scholar]
- 76. Gross G, Huber G, Klosterkoetter J, Linz M.. BSABS. Bonner Skala für die Beurteilung von Basissymptomen (Bonn Scale for the Assessment of Basic Symptoms). Berlin, Germany: Springer, 1987. [Google Scholar]
- 77. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17(4):555–564. [DOI] [PubMed] [Google Scholar]
- 78. Chapman LJ, Edell WS, Chapman JP. Physical anhedonia, perceptual aberration, and psychosis proneness. Schizophr Bull. 1980;6(4):639–653. [DOI] [PubMed] [Google Scholar]
- 79. McGlashan, TH. Structured Interview for Prodromal Symptoms (SIPS). New Haven, CT: Yale University, 2001. [Google Scholar]
- 80. Yung AR, Yuen HP, McGorry PD, et al. . Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39(11-12):964–971. [DOI] [PubMed] [Google Scholar]
- 81. Raballo A, Preti A. Temporal stability of self-disorders and longitudinal unfolding of symptom dimensions: a complementary analysis. Schizophr Res. 2018;195:78–79. [DOI] [PubMed] [Google Scholar]
- 82. Raballo A, Preti A. The self in the spectrum: a closer look at the temporal stability of self-disorders in schizophrenia. Psychopathology. 2018;51(4):285–289. [DOI] [PubMed] [Google Scholar]
- 83. Raballo A, Larøi F. Murmurs of thought: phenomenology of hallucinatory consciousness in impending psychosis. Psychosis. 2011;3(2):163–166. [Google Scholar]
- 84. Parnas J. A disappearing heritage: the clinical core of schizophrenia. Schizophr Bull. 2011;37(6):1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Henriksen MG, Parnas J. Clinical manifestations of self-disorders and the Gestalt of schizophrenia. Schizophr Bull. 2012;38(4):657–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Raballo A. From perception to thought: a phenomenological approach to hallucinatory experience. Schizophr Bull. 2017;43(1):18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Holzman PS. Seymour S. Kety and the genetics of schizophrenia. Neuropsychopharmacology. 2001;25(3):299–304. [DOI] [PubMed] [Google Scholar]
- 88. Poletti M, Gebhardt E, Kvande MN, Ford J, Raballo A. Motor impairment and developmental psychotic risk: connecting the dots and narrowing the pathophysiological gap. Schizophr Bull. 2019;45(3):503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Poletti M, Raballo A. Developmental psychotic risk: toward a neurodevelopmentally informed staging of vulnerability to psychosis. Harv Rev Psychiatry. 2020;28(4):271–278. [DOI] [PubMed] [Google Scholar]
- 90. Raballo A, Poletti M. Advances in early identification of children and adolescents at risk for psychiatric illness. Curr Opin Psychiatry. 2020;33(6):611–617. [DOI] [PubMed] [Google Scholar]
- 91. Schultze-Lutter F, Klosterkotter J, Picker H, Steinmeyer EM, Ruhrmann S. Predicting first-episode psychosis by basic symptom criteria. Clin Neuropsychiatry. 2007;4:11–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.