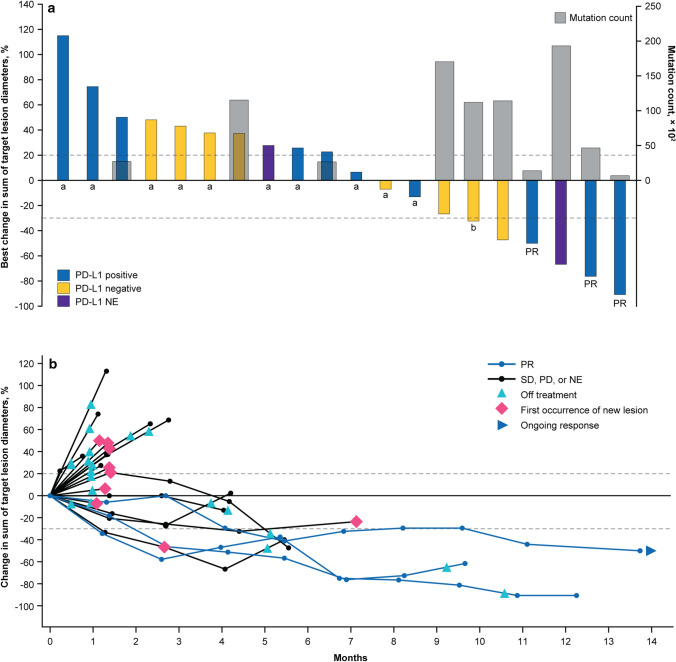
Fig. 1.
Tumor response to bintrafusp alfa assessed by independent review. a Best change in sum of diameters and tumor mutation count. A threshold of 1% was used to characterize tumors as either PD-L1 positive (≥ 1%) or negative (< 1%) using an anti–PD-L1 antibody clone 73-10. Three patients had non-evaluable PD-L1 expression. b Time to and duration of response. The upper dashed line represents progression at 20% increase in size of target lesions, and the lower dashed line represents the RECIST boundary for PR at 30% decrease in size of target lesions. Ten patients are not shown due to having either no target lesions identified by independent review committee prior to the first dose (n = 6), no post-baseline assessment (n = 2), or other reasons (n = 2). NE not evaluable, PD progressive disease, PD-1 programmed cell death protein 1, PD-L1 programmed death-ligand 1, PR partial response, RECIST Response Evaluation Criteria in Solid Tumors, SD stable disease. aTumor mutation count unavailable. bPatient had a best change in sum of diameters of > 30% that did not meet the criteria for a PR at the next tumor assessment