Figure 2. Engraftment of ABE8e-NRCH mRNA-treated SCD patient CD34+ HSPCs after transplantation into immunodeficient mice.
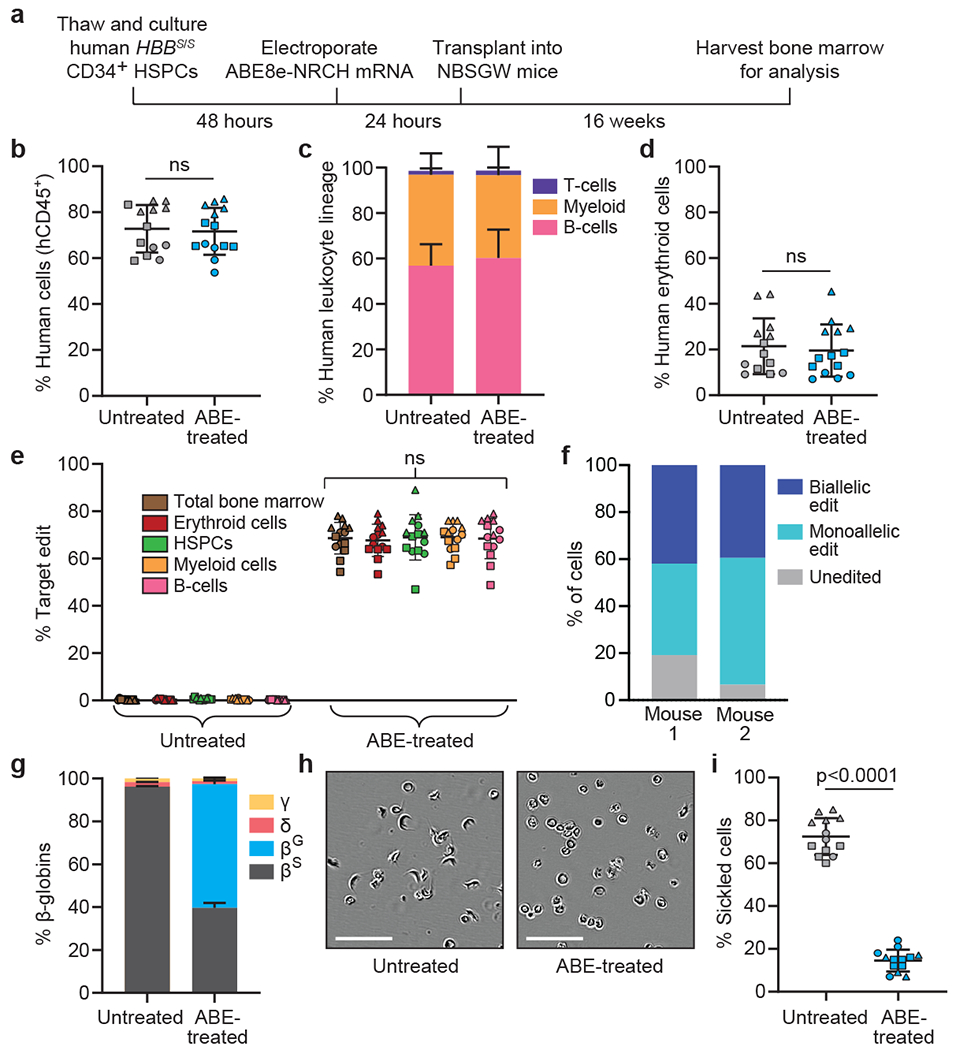
CD34+ HSPCs from three HBBS/S SCD patient donors were electroporated with ABE8e-NRCH mRNA and sgRNA targeting the SCD mutant HBB codon. 2-5x105 treated cells were transplanted into NBSGW mice via tail-vein injection. Mice were analyzed 16 weeks after transplantation. (a) Experimental workflow. (b) Engraftment measured by percentage of human CD45+ (hCD45+) cells in recipient mouse bone marrow. (c) Human B-cells (hCD19+), myeloid cells (hCD33+), and T-cells (hCD3+) cells in recipient mouse bone marrow shown as percentages of the hCD45+ population. (d) Human erythroid precursors (hCD235a+) in recipient mouse bone marrow shown as percentage of human and mouse CD45− cells, (e) HBBS-to-HBBG editing efficiencies in human donor CD34+ cell-derived lineages from recipient bone marrow. Erythroid, myeloid, B-cell, and HSPC human lineages were collected using antibodies that recognize hCD235a, hCD33, hCD19, and hCD34, respectively, (f) Clonal editing outcomes determined by single-cell 5’ RNA-seq in CD235a+ cells from the bone marrow of two edited mice. (g) Proportions of β-like globin proteins by HPLC of human donor-derived reticulocytes isolated from recipient mouse bone marrow. (h) Representative phase-contrast images of human reticulocytes from bone marrow incubated 8 hours in 2% O2. Nine images of >50 cells each were collected per sample. Scale bar=50 μm. (i) Quantification of sickled cells as in Fig. 1e. n=14 mice receiving edited cells and n=13 mice receiving unedited cells in b-e, g, and i. Triangle, square, and circle symbols represent HSPCs from three different SCD donors. Plotted values and error bars reflect mean±SD. Statistical significance was assessed by one-way ANOVA in i and by two-tailed Student’s t-test elsewhere; “ns”, not significant.
