Abstract
There is a worldwide concern regarding the antimicrobial resistance and the inappropriate use of antifungal agents, which had led to an ever-increasing antifungal resistance. This study aimed to identify the antifungal susceptibility of colonized Candida species isolated from pediatric patients with cancer and evaluate the clinical impact of antifungal stewardship (AFS) interventions on the antifungal susceptibility of colonized Candida species. Candida species colonization was evaluated among hospitalized children with cancer in a tertiary teaching hospital, Shiraz 2017–2018. Samples were collected from the mouth, nose, urine, and stool of the patients admitted to our center and cultured on sabouraud dextrose agar. The isolated yeasts identified by polymerase chain reaction–restriction fragment length polymorphisms (PCR–RFLP). DNA Extracted and PCR amplification was performed using the ITS1 and ITS4 primer pairs and Msp I enzyme. The broth microdilution method was used to determine the minimum inhibitory concentrations (MICs) for amphotericin B, caspofungin, and azoles. The prevalence of Candida albicans in the present study was significantly higher than other Candida species. Candida albicans species were completely susceptible to the azoles. The susceptibility rate of C. albicans to amphotericin B and caspofungin was 93.1% and 97.1%, respectively. The fluconazole MIC values of Candida albicans decreased significantly during the post-AFS period (P < 0.001; mean difference: 72.3; 95% CI of the difference: 47.36–98.62). We found that 52.5% (53/117) of the isolated C. albicans were azole-resistant before AFS implementation, while only 1.5% (2/102) of the isolates were resistant after implementation of the AFS program (P < 0.001). C. albicans fluconazole and caspofungin resistant rate also decreased significantly (P < 0.001) after implementation of the AFS program [26 (32.9%) versus 0 (0.0%) and 11 (10.9%) versus 1 (0.9%), respectively]. Besides, fluconazole use (p < 0.05) and fluconazole expenditure reduced significantly (about one thousand US$ per year) after the AFS program. Our results confirm the positive effect of optimized antifungal usage and bedside intervention on the susceptibility of Candida species after the implementation of the AFS program. C. albicans and C. glabrata exhibited a significant increase in susceptibility after the execution of the AFS program.
Subject terms: Cancer, Microbiology
Introduction
The prevalence of candidemia/invasive candidiasis (IC) is on the rise due to excessive usage of broad-spectrum antibiotics, indwelling catheters, HIV infection, malignancies, transplants, invasive procedures, and prolonged hospitalization, especially in intensive care patients and neonates1–3. More than 30 Candida spp. are recognized that they could infect humans4. Overall, 90% of IC are related to C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei5,6.
Antifungal resistance usually occurs following selective pressure induced by the use or misuse of antifungal agents in high-risk patients, especially those with malignancy7–9. The epidemiology of IC could be affected by the type and duration of previous antifungal exposure, such as prolonged antifungal prophylaxis10.
Currently, the urgent need for an AFS program is well recognized and encouraged by many experts11,12. By optimizing antifungal use, including improving the selection and duration of antifungal therapy, potential economic saving also could be achieved12,13. These efforts objectively have been evaluated by different instruments such as total antifungal prescriptions, which defined by daily doses (DDDs) and days of therapy (DOTs)12; however, long term effects of AFS interventions such as potential effects on the epidemiology and the antifungal susceptibility patterns are less known. Although C. albicans is the most common cause of IC, the prevalence of non-albicans species increases7. The emergence of non-albicans Candida infections has become a global concern; however, as we described previously, change in the epidemiologic pattern could be possible after sustained adherence to the AFS program14. Similar positive effects could be expected on the susceptibility pattern of Candida species after AFS implementation. Therefore, this study aimed to identify the antifungal susceptibility of colonized Candida species isolated from pediatric patients with malignancy and investigate the ASP intervention effects on the antifungal susceptibility patterns.
Methods
Study design
This is a cross-sectional study investigating the susceptibility pattern of colonized Candida species in children with malignancy. Samples were collected from oral/nasal secretions and urine/stool specimens. Every eligible patient undergoes regular weekly sampling after admission until discharge. We used the original data from our previous study in Amir medical oncology center (AMOC), which was conducted before the implementation of AFS during 2011–2012 in colonized pediatric patients with malignancy (period-1; p1)15 to compare the clinical impact of AFS interventions on the antifungal susceptibility of colonized Candida species with our present study (period-2; p2). So, it should be mentioned that this study was designed to investigate the susceptibility of colonized Candida species before and after the implementation of AFS in a referral tertiary oncology center.
Participants
In this study, children aged < 18-year-old with hematologic malignancy or solid organ tumors were included between 2017 and 2018. In children with severe thrombocytopenia or bleeding tendency, only urine and stool samples were collected.
Mycological study
Samples were cultured on Sabouraud Dextrose Agar (Merck, Germany) medium and transferred to the mycology laboratory of Professor Alborzi Clinical Microbiology Research Center for identification and susceptibility testing. The isolated yeasts identified by polymerase chain reaction–restriction fragment length polymorphisms (PCR–RFLP)16. DNA Extracted and PCR amplification was performed using the ITS1 and ITS4 primer pairs (MWG-Biotech AG, Germany) and Msp I enzyme17. The isolated fungi were cultured twice on Potato Dextrose Agar (OXOID LTD, Basingstoke, Hampshire, England) medium at 35 °C for 24–48 h to ensure the purity of the isolates. C. parapsilosis ATCC-22019 and C. krusei-ATCC-6258 were used as standard quality control CLSI-recommended strains.
Antifungal susceptibility testing
The susceptibility testing of amphotericin B (AMB) and posaconazole (POS) (Sigma-Aldrich, Germany), caspofungin (CAS) fluconazole (FLU), itraconazole (ITR) and voriconazole (VOR) (Sigma-Aldrich, USA) were performed according to CLSI M27-A318 and CLSI M27-S419.
Briefly, RPMI 1640 medium (Sigma-Aldrich, England) with l-glutamine and 2% glucose was prepared. PH adjusted to 7.0. Inoculum’s suspension of each yeast (0.5 McFarland) was prepared using the spectrophotometric method at 530 nm. Serial dilution with RPMI was prepared for fluconazole from 0.125 to 64 μg/mL and other antifungal agents from 0.032 to 16 μg/m. Positive and negative control (wells without antifungals and wells without yeast) were considered for evaluating the tests. The MIC was read visually after 24 and 48 h. The MIC for POS, CAS, FLU, ITR, and VOR were described as the lowest concentration of antifungal agent could decrease fungal growth by 50% compared to positive controls. For AMB, complete growth inhibition was considered as MIC value. The wild-type species is a sensitive species that presents no mutation or acquires antifungal resistant gen. In resistant species (non-wild type), there is some resistant gen that exhibits a high MIC value. Epidemiological cut-off value (ECV) is defined as the MIC value at least 95% of wild-type isolates under this MIC value20,21. The MIC50, MIC90, and ECV of the isolated species and wild and non-wild species were calculated.
Antifungal Stewardship program in Amir medical oncology center
AFS is a “strategic planning” that can be summarized in learning, training, and continuous practice to improve evidence-based skills in managing invasive fungal diseases (IFDs), including IC in high-risk patients. By the sustained adherence to the AFS, indiscriminate use of antifungal agents, drug resistance, side effects, and costs will be reduced. The AFS has been executed in our center since June 2015. Characteristics of AFS interventions are summarized in Table 1. It should be noted that the diagnosis and treatment of the IFDs were significantly improved after the implementation of the AFS. Changing from empiric therapy to pre-emptive antifungal treatment strategies was accomplished by the application of non-culture-based methods, such as galactomannan (GM) antigen, mannan, and polymerase chain reaction (PCR). Therapeutic drug monitoring and antifungal susceptibility testing have become the standard of care for monitor serum voriconazole concentrations and targeted therapy since early 2016.
Table 1.
Main components of AFS interventions for the management of invasive fungal diseases (including invasive candidiasis and invasive aspergillosis) in Amir medical oncology center.
| Appropriate treatment of the suspected IFDs |
| Disposition to targeted therapy (by diagnostic driven approach) instead of empiric treatment |
| Adherence to current evidence-based guidelines in the treatment of the IFDs instead of individual decision making |
| Appropriate antifungal prescription |
| Appropriate antifungal selection |
| Appropriate duration |
| Appropriate administration route |
| Appropriate dosage |
| Limited use of azoles for prophylaxis of the IFDs (only for secondary prophylaxis in patients with a previous history of IFDs) |
| Regular epidemiologic surveillance to estimate of fungal infection incidence and detection of any epidemiologic shift |
| Regular surveillance of the susceptibility pattern to antifungal drugs |
| Appropriate use of new diagnostic modalities (implementation of routine GM test, twice/week during prolonged and profound neutropenic phase (ANC < 500 cells/mm3) |
| Improving mycological diagnostic approach with judicious use of bronchoalveolar lavage and ultrasound/CT scan guided lung biopsy (or other organs as needed) |
| Time-sensitive automatic stop orders for specified antifungal prescriptions |
| Switching from intravenous to oral antifungal, when appropriate and confirmed by the infectious disease consultant |
| Full-time laboratory services (24-h, 7 days per week coverage) and strategies for reducing lab turnaround time (establishing a “hotline” for contributors to call about the lab test results) |
| Non-medical approach to prevent fungal infections |
| Applying modalities to reduce the nosocomial infections (for example, diminished colonization by the appropriate use of an indwelling catheter) |
| Surveillance of the possible environmental roots of infection (for example, surveillance of indoor spore load in the hospital’s wards) |
AFS antifungal Stewardship, IFDs invasive fungal diseases, IMDs invasive mold diseases, GM galactomannan, ANC absolute neutrophil count, AMOC Amir Medical Oncology Center.
As we know, if the colonized fungi population contains some resistance strains, they will show resistance if exposed to antifungal drugs. In heterogenous fungi population after exposure to antifungal medications, resistance could be acquired by selection pressure7. Amphotericin will be the main culprit for antifungal prophylaxis, while fluconazole use has dropped dramatically during the second study period. Non-azole antifungal prophylaxis was implemented in our center to save last-line azole agents (voriconazole and posaconazole) for treating invasive mold infections. We test that our prophylaxis strategy could affect amphotericin resistance rate during the second study period.
Statistical analysis
Data were analyzed using IBM SPSS Statistics 21 software (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). All categorical variables reported in percentages and numbers. P values calculated using the Chi-square test and Fisher’s exact test. P values < 0.05 considered being statistically significant. The Pearson correlation test was used to investigate the correlations between quantitative variables.
Ethics and consent to participate
The study was approved by the medical ethics committee of Professor Alborzi clinical microbiology research center, Shiraz University of medical sciences, Iran (ID number: 94-01-49-11275). The authors confirm that all methods performed in accordance with the relevant guidelines and regulations. All individuals (or their parents) in the study population were informed about the current study, with written consents obtained before enrolment in the present study.
Results
The incidence of IFDs was ranged from 7.7 to 12.5/1000 admissions during 2015–2018 in our center. Invasive candidiasis (IC) is the most common form of IFDs (47.2%), and its annual incidence range is 22.5–55.3%.
From May 2017 to November 2018, 482 specimens were collected from 136 pediatric patients with hematological malignancies or solid organ tumors. Most patients were male (53.3%), and the mean age was 7.57 years (Median: 6.5, Std. Deviation: ± 4.85, range from 4.8 months to 18 years). During this period, 36% of the studied cases were monitored for at least 4 weeks by weekly sampling, whereas 64% followed for more than four weeks.
Acute lymphoblastic leukemia (41/136, 30.1%), acute myeloblastic leukemia (18/136, 13.2%), and neuroblastoma (13/136, 9.5%) were the most common underlying diseases, respectively. In total, 51.4% (70) were neutropenic (absolute neutrophil count < 1500 cells/mm3).
Eighty-two cases were colonized with at least one Candida spp. and 133 strains of Candida species identified (two species not identified). The most prevalent isolated species was C. albicans (102 strains) followed by C. krusei (7), C. kefyr (7), C. parapsilosis (5), C. glabrata (4), C. tropicalis (3), and C. famata (3). The susceptibility of Candida species to different antifungal drugs summarized in Table 2.
Table 2.
Susceptibility of 131 Candida spp. to antifungal drugs and distributions of MIC (µg/ml) by CLSI broth microdilution method.
| Organism | AF | Breakpoints | S | SDD | I | R | ECVa | WT | N-WT | MIC50a | MIC90a | MIC rangea |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. albicans | AmpB | S ≤ 1, R ≥ 1 | 93.1% | – | – | 6.9% | 4 | 96% | 4% | 0.250 | 0. 50 | 0.032–8 |
| CSF | S ≤ 0.25, I = 0.5, R ≥ 1 | 97.1% | – | 1.96% | 1% | 0.25 | 97% | 3% | 0.032 | 0.064 | 0.032–1 | |
| VCZ | S ≤ 0.12, I = 0.25, − 0.5 R ≥ 1 | 100% | – | – | – | 0.032 | 98% | 2% | 0.032 | 0.032 | 0.032–0.125 | |
| FCZ | S ≤ 2, SDD = 4, R ≥ 8 | 100% | – | – | – | 0.25 | 98% | 2% | 0.032 | 0.125 | 0.032–4 | |
| ITC | S ≤ 0.12, SDD = 0.25, − 0.5 R ≥ 1 | 100% | – | – | – | 0.064 | 98% | 2% | 0.032 | 0.032 | 0.032–0.064 | |
| C. glabrata | AmpB | S ≤ 1, R ≥ 1 | 100% | – | – | – | 0.25 | 75% | 25% | 0.250 | 0.5 | 0.25–0.5 |
| CSF | S ≤ 0.12, I = 0.25, R ≥ 0.5 | 75% | – | 25% | – | 0.125 | 75% | 25% | 0.125 | 0.25 | 0.064–0.25 | |
| VCZ | ECV = 0.5, WT: MIC ≤ ECV & non-WT: MIC > ECV | 0.032 | 100% | – | 0.032 | 0.032 | 0.032 | |||||
| FCZ | SDD ≤ 32, R ≥ 64 | – | 100% | – | – | 0.25 | 75% | 25% | 0.25 | 1 | 0.125–1 | |
| ITC | S ≤ 0.12, SDD = 0.25, − 0.5 R ≥ 1 | 100% | – | – | – | 0.064 | 75% | 25% | 0.064 | 0.125 | 0.064–0.125 | |
| C. krusei | AmpB | S ≤ 1, R ≥ 1 | 100% | – | – | – | 0.5 | 85.7% | 28.6% | 0.5 | 1 | 0.25–1 |
| CSF | S ≤ 0.25, I = 0.5, R ≥ 1 | 14.3% | – | 57.1% | 28.6% | 0.5 | 71.4% | 14.3% | 0.5 | 1 | 0.25–1 | |
| VCZ | S ≤ 0.5, I = 1, R ≥ 2 | 100% | – | – | – | 0.125 | 85.7% | 28.6% | 0.125 | 0.25 | 0.064–0.25 | |
| FCZ | C. krusei is considered resistant to FCZ, irrespective of the MIC | – | – | – | – | – | – | |||||
| ITC | S ≤ 0.12, SDD = 0.25, − 0.5 R ≥ 1 | 85.7% | 14.3% | – | – | 0.125 | 85.7% | 28.6% | 0.125 | 0.25 | 0.125–0.25 | |
| C. tropicalis | AmpB | S ≤ 1, R ≥ 1 | 100% | – | – | – | 0.25 | 66.7% | 33.3% | 0.25 | 0.5 | 0.25–0.5 |
| CSF | S ≤ 2 | 100% | – | – | – | 0.064 | 66.7% | 33.3% | 0.064 | 1 | 0.032–1 | |
| VCZ | S ≤ 0.12, I = 0.25, − 0.5 R ≥ 1 | 100% | – | – | – | 0.032 | 100% | – | 0.032 | 0.032 | 0.032 | |
| FCZ | S ≤ 8, R ≥ 64 | 100% | – | – | – | 0.032 | 66.7% | 33.3% | 0.032 | 0.125 | 0.032–0.25 | |
| ITC | S ≤ 0.12, SDD = 0.25, − 0.5 R ≥ 1 | 100% | – | – | – | 0.032 | 100% | – | 0.032 | 0.032 | 0.032 | |
| C. parapsilosis | AmpB | S ≤ 1, R ≥ 1 | 100% | – | – | – | 0.25 | 80% | 20% | 0.25 | 0.5 | 0.032–0.5 |
| CSF | S ≤ 2, I = 4, R ≥ 8 | 100% | – | – | – | 0.5 | 80% | 20% | 0.064 | 0.125 | 0.032–0.125 | |
| VCZ | S ≤ 0.12, I = 0.25, − 0.5 R ≥ 1 | 80% | – | 20% | – | 0.032 | 80% | 20% | 0.032 | 0.5 | 0.032–0.5 | |
| FCZ | S ≤ 2, SDD = 4, R ≥ 8 | 80% | – | – | 20% | 0.064 | 80% | 20% | 0.064 | 16 | 0.032–16 | |
| ITC | S ≤ 0.12, SDD = 0.25, − 0.5 R ≥ 1 | 80% | 20% | – | – | 0.032 | 80% | 20% | 0.032 | 0.25 | 0.032–0.25 | |
Based on recommended CLSI 24-h minimum inhibitory concentration limits.
AmpB Amphotericin B, CSF Caspofungin, VCZ Voriconazole, FCZ Fluconazole, ITC Itraconazole, AF antifungal, SDD susceptible dose-dependent, S sensitive, I intermediate, R resistant, ECV Epidemiological Cutoff Value; ECVs capture ≥ 97.5% of the statistically modelled population, WT Wild-type, NWT non-wild-type, MIC50 Minimum Inhibitory Concentration required to inhibit the growth of 50% of organisms, MIC90 Minimum Inhibitory Concentration required to inhibit the growth of 90% of fungal species.
a(µg/ml).
All C. albicans were susceptible to the azole antifungal agents. The susceptibility rate of C. albicans to amphotericin B and caspofungin was 93.1% (95) and 97.1% (99), respectively. All the C. krusei isolates were sensitive to amphotericin B and voriconazole; while, 28.6% were resistant to caspofungin. For itraconazole, 85.7% were sensitive, and 14.3% were susceptible dose-dependent. C. parapsilosis isolates were sensitive to amphotericin B, caspofungin. For itraconazole, 80% were sensitive, and 20% were susceptible dose-dependent. 80% of C. parapsilosis found to be susceptible to fluconazole. All C. glabrata and C. tropicalis isolates were sensitive to the tested antifungal agents.
The ECV, MIC50, and MIC90 for in vitro susceptibility testing of Candida spp. calculated (Table 2). Susceptibility of different antifungals to C. kefyr and C. famata is provided in Table 3. CLSI breakpoints are not available for C. Kefyr and C. famata.
Table 3.
Susceptibilities of different antifungals to C. kefyr and C. famata.
| Species (no. tested) | Antifungal agent | MIC (μg/ml) | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| C. kefyr (7) | Fluconazole | 0.032–0.25 | 0.064 | 0.125 |
| Voriconazole | 0.032 | 0.032 | 0.032 | |
| Itraconazole | 0.032 | 0.032 | 0.032 | |
| Caspofungin | 0.032–1 | 0.064 | 0.125 | |
| Amphotericin B | 0.064–2 | 0.25 | 0.5 | |
| C. famata (3) | Fluconazole | 0.032–0.25 | 0.032 | 0.032 |
| Voriconazole | 0.032 | 0.032 | 0.032 | |
| Itraconazole | 0.032–0.25 | 0.032 | 0.032 | |
| Caspofungin | 0.032–0.125 | 0.032 | 0.032 | |
| Amphotericin B | 0.25–0.5 | 0.25 | 0.25 | |
CLSI does not provide posaconazole minimal inhibitory concentration breakpoint for C. albicans. Posaconazole 24-h and 48-h MIC statistics were determined for 102 C. albicans isolates. Accordingly, mean 24-h and 48-h MIC were 0.0361 and 0.0394, respectively (Table 4, Fig. 1).
Table 4.
Posaconazole 24-h and 48-h MIC statistics for 102 isolates of C. albicans.
| 24-h MIC | 48-h MIC | |
|---|---|---|
| Mean | 0.0361 | 0.0394 |
| Median | 0.0320 | 0.0320 |
| Mode | 0.03 | 0.03 |
| Std. deviation | 0.02470 | 0.04727 |
| Variance | 0.001 | 0.002 |
| Range | 0.22 | 0.47 |
| Minimum | 0.03 | 0.03 |
| Maximum | 0.25 | 0.50 |
Figure 1.
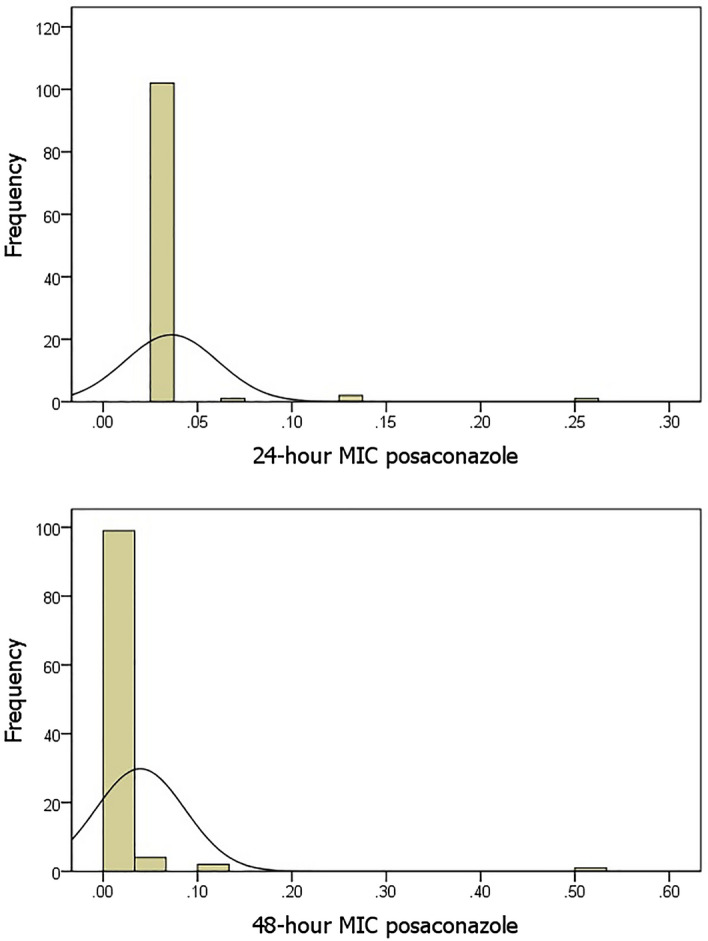
24-h and 48-h MIC distribution with a histogram of the isolated C. albicans.
Epidemiological changes in the Candida colonization pattern described in our previous report. During period 1 (p1), 46.5% (88) of the studied cases (n = 188) were colonized, while in the 2nd period, the colonization rate reached 59.9% (P value = 0. 0.017)14. In total, 25.3% (23) of the cases were receiving inpatient-antifungal prophylaxis during the 2nd period, mainly with the liposomal formulation of amphotericin B, while 54% were on antifungal prophylaxis during p1, mostly with fluconazole or itraconazole [Difference 21.2%, 95% CI 9.16–31.77%, P = 0.0007]. Despite a significant increase in the colonization rate, we found a significant reduction in non-albicans species colonization after the implementation of AFS. This success was achieved by controlling and restricting antifungal usage during p2.
In a study by Hadadi et al., which was conducted during 2011–2012 (p1) in our center, C. albicans was the most common species, followed by C. krusei, C. glabrata, C. tropicalis, C. famata, C. parapsilosis, C. dubliniensis, and C. kefyr. During p1, C. glabrata was the most resistant isolated Candida species, showing 70% resistant to fluconazole and 50% to itraconazole, 7.5% to amphotericin B, and 14% to ketoconazole15.
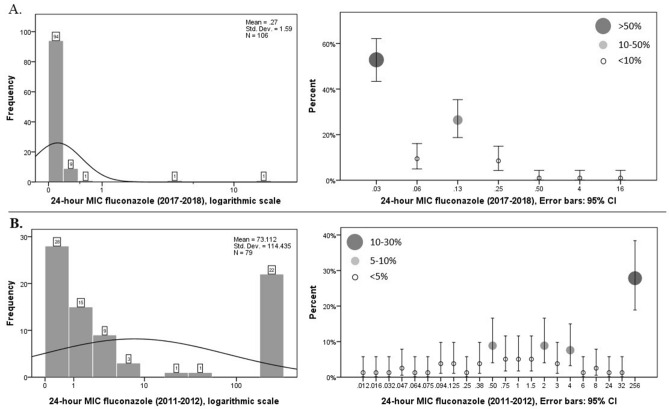
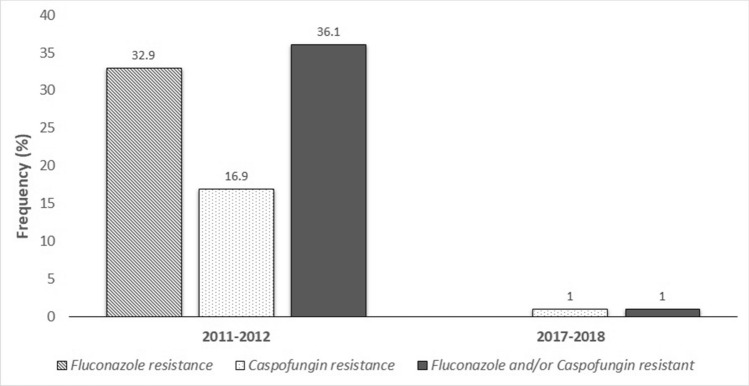
During p1, 52.5% (53/117) of the isolated C. albicans were found azole-resistant, while only 1.5% (2/102) of the isolates were azole-resistant during p2 (P value < 0.001). Amongst the 117 tested isolates of C. albicans, 52.5% (53) of the isolates were found to be azole-resistant during p1, while only 1.5% (2) were resistant during p2 (P value < 0.001). No fluconazole-resistant (MIC ≥ 8 μg/ml) C. albicans was detected during p2 (P value < 0.001). Multidrug-resistant strains, including azole, caspofungin, and amphotericin B resistant isolates, were not found within the two study periods (Fig. 2).
Figure 2.
24-h MIC fluconazole of 117 (2011–2012), and 106 (2017–2018) strains of C. albicans. (A) Illustrate chart bar (left) which each bar is labeled with the number of isolates and logarithmic scales (right) of 24-h MIC fluconazole during p2 (2017–2018) which Frequency of MIC results is presented in error bars with 95% CI. Each error bar is labeled by circles that are representative of MIC frequency. (B) Illustrate chart bar (left) and logarithmic scales (right) of 24-h MIC fluconazole during p1 (2011–2012). MIC distribution histogram also is provided for better comparison between the two periods.
Despite the significant reduction in fluconazole and caspofungin-resistant, during p2, a slight increase in the incidence of amphotericin B-resistant C. albicans was detected (Table 5). This change could be explained by the antifungal preventive strategy shifting to liposomal amphotericin B since 2015. However, the frequency of amphotericin B-resistant C. albicans was not affected by liposomal amphotericin B prophylaxis between the two periods (p = 0.619) (Fig. 3).
Table 5.
The susceptibility of isolated C. albicans against fluconazole, caspofungin, and amphotericin B, during 2011–12 (period 1) and 2017–2018 (period 2).
| Antifungal agent | Susceptibility | Period 1 | Period 2a | p-value |
|---|---|---|---|---|
| Fluconazole | Sensitive | 53 (67.1) | 102 (100) | < 0.001** |
| Resistant | 26 (32.9) | 0 | ||
| Caspofungin | Sensitive | 94 (89.5) | 101 (99.1) | < 0.001** |
| Resistant | 11 (10.9) | 1 (0.9) | ||
| Amphotericin B | Sensitive | 83 (100) | 95 (93.1) | < 0.001** |
| Resistant | 0 | 7 (6.9) |
aNumber (%) of children colonized with C. albicans.
*No fluconazole-resistant isolates of C. albicans was found during period 2 (2017–2018).
**Statistically significant by Fisher’s exact test.
Figure 3.
Frequency of fluconazole-resistant, caspofungin-resistant and fluconazole and/or caspofungin-resistant strains of C. albicans during the two study periods.
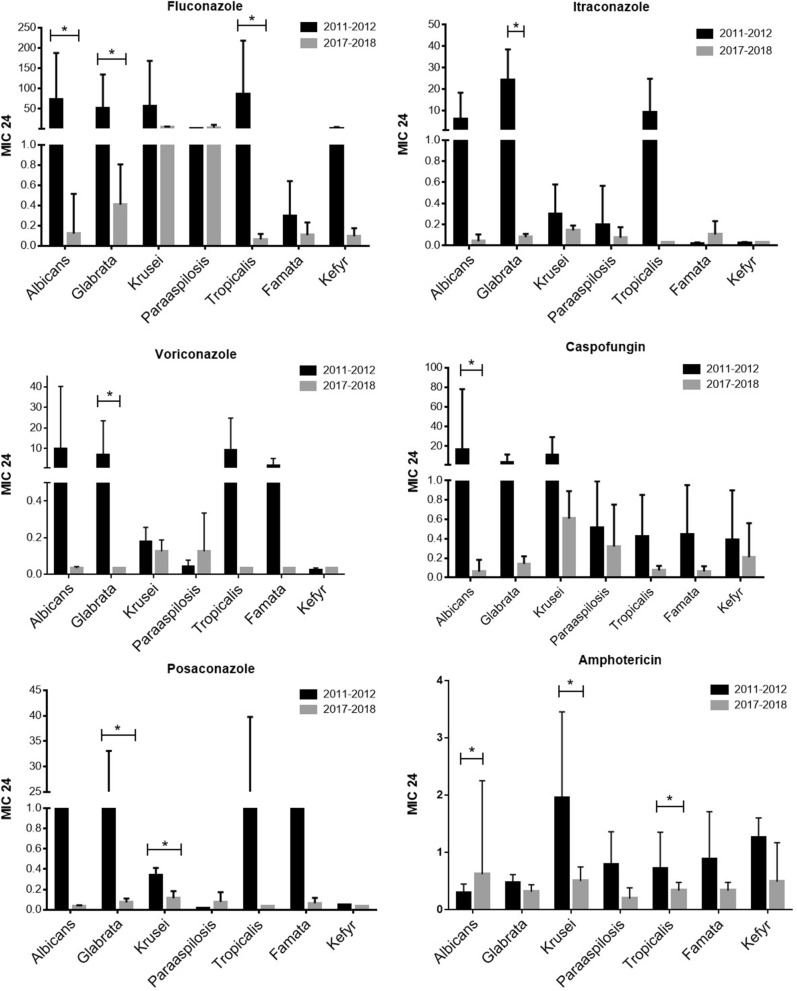
We also analyzed the rate of fluconazole, voriconazole, itraconazole, caspofungin, and amphotericin B resistance amongst the non-albicans colonized species between the two study periods. A significant decrease in fluconazole, itraconazole, and caspofungin resistance was found among the C. glaberata strains during the second study period (p2) compared with 2011–2012. Also, a statistically significant reduction in amphotericin B resistance (p = 0.007) found during p2 in C. krusei isolates (Fig. 4).
Figure 4.
The mean MIC value (24-h) of C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, C. famata, and C. kefyr for fluconazole, itraconazole, voriconazole, caspofungin, posaconazole, and amphotericin B, during the two study periods. Error bars represent standard deviations. *P ≤ 0.05 by the two-way ANOVA test.
We also review our available data for fluconazole usage (including multiple courses of fluconazole prescriptions per patient) before and after implementing the AFS program and their impact on health economics. During the last year before the initiation of the AFS program (2014–2015), fluconazole prescribed for 161 patients (total admissions: 4975), while during the first year of the AFS program (2015–2016), fluconazole administrated in 92 cancer patients (total admissions: 5706). The fluconazole consumption showed significant decrease (p < 0.001) from 3.2 to 1.6% (33 in 1000 cases to 16 in 1000 cases). No significant changes observed in the crude mortality rate after implementing the AFS program (0.7% versus 0.5%, respectively; p = 0.471). The total cost of the fluconazole usage was also reduced by 610 US$ after the start of the AFS program (6099 US% versus 5189 US%, p = 0.164). Notably, mean days of prescription for each patient who received fluconazole increased during p2 to 36/1000 patients (3.37 days; SD: ± 2.67) compare to the p1 16/1000 patients (2.58 days; SD: ± 3.035), which is statistically significant (p = 0.0315).
Discussion
Amongst the 136 studied cases, 60% were colonized with at least one Candida species. Most of them were colonized with C. albicans, while C. krusei, C. kefyr, C. glaberata, C. parapsilosis, C. tropicalis, and C. famata were the least common Candida species. Our finding is in agreement with other reports on Candida colonization in children with malignancy22–24. Detailed information regarding the colonization pattern of the studied cases can be found in our recently published paper14.
Most Candida bloodstream infections, including central line-associated candidemia, originate from endogenous host flora25–27. The clinical impact of Candida colonization on the short-term mortality rate of patients with hematological malignancies has been documented in previous reports28,29. Higher mortality rates have been detected in patients with non-albicans species, such as C. glabrata, C. kefyr, and C. krusei, compared with C. albicans28,30. As discussed earlier, during p1, more than 35% of cases were colonized with non-albicans species, mostly C. glabrata and C. krusei. However, after implementing the AFS, non-albicans colonization decreased to less than 20%, mostly C. krusei and C. Parapsilosis, with a significant decrease in C. glabrata colonization14. C. glabrata is considered the second most common gastrointestinal yeast flora after C. albicans31. While an epidemiological shift from C. albicans to non-albicans species has been observed mainly in patients with hematological malignancies32, our recent survey confirmed that the successful implementation of AFS programs could reverse this shift.
We found full azole susceptibility of the isolated C. albicans in addition to 99% and 93% susceptibility to caspofungin and amphotericin B, respectively. Compare to other reports from our region; this study showed better susceptibility of colonized C. albicans to fluconazole and caspofungin9,24. Our finding confirmed that the AFS program (including amphotericin prophylactic strategy) could save azole antifungals as a first-line choice for IC.
In the present study, all clinical isolates of C. krusei, C. glaberata, C. parapsilosis, and C. tropicalis isolates were susceptible to amphotericin B (the most active agent for the treatment of non-albicans Candida species). Notably, despite our changing prophylactic strategy, much better susceptibility to amphotericin B was detected for isolated C. krusei as the most common non-albicans Candida species. Similar studies in our country shown 38.5–40% resistance to isolated C. krusei in colonized patients9,24. We found a higher resistance rate against caspofungin in isolated C. krusei (28.6%) compare to the previous reports in different parts of Iran9,15,24,33. The emergence of echinocandin-resistant C. krusei may be a paramount concern given the high MIC to fluconazole and voriconazole32.
Accordingly, amphotericin B can be considered the most active agent for treating non-albicans Candida species, especially C. krusei and C. glaberata in our study. Also, in this study, the colonized isolates of C. Kefyr and C. famata were susceptible to the tested antifungal agents.
In addition to the susceptibility results, we also compared the mean MIC value of each antifungal drug for C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, C. famata, and C. kefyr during two study periods (Fig. 4). As shown in Fig. 4, a significant reduction in mean FCZ-MIC found for C. albicans, C. glabrata, and C. tropicalis in p2 compare to p1.
Based on our obtained results, all C. albicans isolates were susceptible to the tested azoles. Besides, these clinical isolates showed high susceptibility to amphotericin B and caspofungin (93.1% and 97.1%, respectively). Compare to the previously reported C. albicans susceptibility, which performed on the various clinical samples collected during 2005–2010, in Shiraz; higher azole-susceptibility was found in this study for C. albicans isolates, while susceptibility to amphotericin B and caspofungin (93% and 98.2%, respectively)6 remained unchanged.
At a global level, in some regions such as South Africa (African Region) and Taiwan, China (Western Pacific Region), fluconazole resistance C. albicans more frequently reported34. Fluconazole resistance C. albicans could be considered a predictor of cross‐resistance between azoles, especially in those with prior exposure to this antifungal class35,36. Cross-resistant between azoles and echinocandins among Candida species is another concern37,38.
As we have shown in this report, stewardship program is an efficacious approach for optimizing the use of antifungal drugs and improving azole susceptibility against Candida species, which could be achieved successfully by judicious AFS guideline adherence and facility-specific treatment recommendation monitoring.
As we mentioned earlier in the results section, the frequency of amphotericin B-resistant C. albicans was not affected by liposomal amphotericin B prophylaxis between the two periods. Our finding is promising compared with other reports concerning the change in resistance patterns of Candida species from fluconazole resistance to echinocandins resistance and the emergence of multidrug-resistant Candida species by increased therapeutic use of echinocandins39. The emergence of azole-resistant C. glabrata is also a concern in the setting that uses fluconazole prophylaxis10. In addition, resistance to amphotericin B remains relatively uncommon among Candida species40,41.
IFD continues to make a substantial economic burden on the oncology centers42. Many reports confirmed the benefits of AFS programs on the IFD-attributable hospital costs and reducing toxicities of antifungal agents13. Although, the clinical impact of AFS on the susceptibility of invasive fungi has not been investigated thoroughly, especially in high-risk cancer patients11,43. Given the emergence of antifungal resistance Candida species, appropriate use of antifungals and implementation of AFS programs is of utmost importance.
Therapeutic options for fungal infections are limited even before the global rise of antifungal resistance34,44; hence, a judicious prescription of available choices, especially non-azole antifungals, should be considered in high-risk settings, such as oncology centers. As we summarized in Table 1, our AFS program contains different strategies for optimizing antifungal drug prescription in patients suspicious of invasive forms of candidiasis and aspergillosis. Some examples are mentioned here for a better explanation. Before the beginning of the AFS program, febrile neutropenic patients universally receiving empiric antifungal therapy after 3–5 days of sustained fever without judicious use of state-of-the-art available diagnostic tests, including non-invasive tests such as automated blood culture systems, specific none culture-based mycologic assays (such as fungal polymerase chain reaction, galactomannan, and mannan) and interventional diagnostic modalities such as bronchoscopy/bronchoalveolar lavage, CT/ultrasound-guided lung biopsy, and sinus/skin biopsy. Indeed, our approach to febrile neutropenia changed from an empiric approach to a diagnostic-driven approach or pre-emptive treatment as suggested by experts and newer guidelines45–50. As we mentioned earlier in the result section, fluconazole prescription decreased per patient/1000 admission/year during the post-AFS period, but with the correct dose and duration. It should be noted that the AFS interventions should not put the cancer patient at greater risk of IFD, and a wise prescription of AF agents (formulary restrictions) should be weighed against high case-fatality rates of IFDs.
Our antifungal prophylaxis strategy changed after June 2015 to the liposomal formulation of amphotericin B. Particularly, antifungal prophylaxis alone is not fully effective without using air filtration system through high-efficiency particulate air filtration (HEPA) filters51; however, due to limited financial resources for providing HEPA filters, on-going hospital construction, potential risk of azole-resistant fungi, limited available new-azoles (posaconazole, isavuconazole, and ravuconazole) and echinocandins (micafungin and anidulafungin), and also increased number of invasive mucormycosis52, AFS team decide to use liposomal amphotericin B for antifungal prophylaxis. It should be reemphasized that amphotericin is routinely not recommended as systemic antifungal prophylaxis53; however, it should be noticed that liposomal amphotericin is not included in studies comparing amphotericin versus fluconazole53 and, so, a liposomal formulation of amphotericin B may be used in high-risk pediatric patients recommended by guidelines47,50. Besides, azole prophylaxis has a critical role in developing either unsusceptible strains or selecting intrinsic azole-resistant yeasts, such as C. krusei7,10,54,55.
In addition to the strategy mentioned above, we found that fluconazole had overused for treatment of the fungal mucositis (as one of the most common infectious complications during or after chemotherapy) which successfully replaced with nystatin and amphotericin-B mouth wash in non-severe cases (WHO grade I and II) who tolerate gargling. Prevention of unnecessarily prolonged catheterization and implementing bundled strategies for preventing central line-associated bloodstream infections (CLABSI) are other examples for preventing IC in our center.
There are lots of data concerning the positive effect of stewardship programs on bacterial resistance56–59; however, antifungal resistance is more challenging to measure due to its multi-factorial development. Even in colonized patients, susceptibility patterns might change over time, especially in immunocompromised hosts9. Although the AFS program has known short-term effects (e.g., reduction in antifungal consumption, costs, and outcomes) on the management of IFDs and patient safety13,43,60,61, its long-term effects have been described on resistance patterns62. Based on stewardship program metrics, change in resistance patterns and pathogen-specific resistance is the most challenging target56. There are scarce reports on the improvement of antifungal susceptibility of Candida species overtimes after the implementation of the AFS program to the best of our knowledge. Hence, the results of our study highlight the importance of strict adherence to the stewardship programs amongst cancer patients.
The small number of samples limited this study. Additionally, further studies using next-generation sequencing are needed to detect AFS program effects on antifungal resistance genes in Candida species.
In conclusion, C. albicans are the most prevalent colonizer among pediatric patients with malignancy, and azoles remain the most effective choice when used wisely. Improving Candida species antifungal susceptibility after the implementation of AFS is promising. Knowledge of etiologic agents and the regular identification of antifungal susceptibility patterns are necessary for oncology settings.
Acknowledgements
The authors wish to thank Mr. H. Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript.
Author contributions
Study concept and design: A.A. and H.S.S.; Acquisition of data: A.A., J.H., H.S.S. and G.F.; Mycological analysis: B.P., J.H. and G.F.; Statistical Analysis: A.A., H.S., and N.S., Analysis and interpretation of data: A.A. and H.S.S.; Drafting of the manuscript: A.A., Critical revision of the manuscript for important intellectual content: A.A. and B.P.; Study supervision: A.A. and B.P. All individuals listed as (co)-authors have met the authorship criteria, and nobody who qualifies for authorship has been omitted from the list. The final manuscript was corrected and approved by all authors.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Parisa Badiee, Email: badieep@gmail.com.
Seyedeh Sedigheh Hamzavi, Email: s.hamzavi55@yahoo.com.
References
- 1.Richardson M, Lass Flörl C. Changing epidemiology of systemic fungal infections. Clin. Microbiol. Infect. 2008;14:5–24. doi: 10.1111/j.1469-0691.2008.01978.x. [DOI] [PubMed] [Google Scholar]
- 2.Brown GD, et al. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 3.Sweileh WM, Sawalha AF, Al-Jabi S, Zyoud SEH. Bibliometric analysis of literature on antifungal triazole resistance: 1980–2015. Germs. 2017;7:19. doi: 10.18683/germs.2017.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarti A, et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2015;41:285–295. doi: 10.1007/s00134-014-3603-2. [DOI] [PubMed] [Google Scholar]
- 5.Zand F, et al. Invasive fungal infections in critically-ill patients: A literature review and position statement from the IFI-clinical forum, Shiraz, Iran. Biosci. Biotechnol. Res. Commun. 2016;9:371–381. doi: 10.21786/bbrc/9.3/6. [DOI] [Google Scholar]
- 6.Badiee P, Alborzi A. Susceptibility of clinical Candida species isolates to antifungal agents by E-test, Southern Iran: A five year study. Iran. J. Microbiol. 2011;3:183. [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen RH. Resistance in human pathogenic yeasts and filamentous fungi: Prevalence, underlying molecular mechanisms and link to the use of antifungals in humans and the environment. Dan. Med. J. 2016;63:1–34. [PubMed] [Google Scholar]
- 8.Gonçalves SS, Souza ACR, Chowdhary A, Meis JF, Colombo AL. Epidemiology and molecular mechanisms of antifungal resistance in Candida and Aspergillus. Mycoses. 2016;59:198–219. doi: 10.1111/myc.12469. [DOI] [PubMed] [Google Scholar]
- 9.Badiee P, et al. Antifungal susceptibility testing of Candida species isolated from the immunocompromised patients admitted to ten university hospitals in Iran: Comparison of colonizing and infecting isolates. BMC Infect. Dis. 2017;17:727. doi: 10.1186/s12879-017-2825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kullberg BJ, Arendrup MC. Invasive candidiasis. N. Engl. J. Med. 2015;373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz P, Bouza E. The current treatment landscape: the need for antifungal stewardship programmes. J. Antimicrobial Chemother. 2016;71:ii5–ii12. doi: 10.1093/jac/dkw391. [DOI] [PubMed] [Google Scholar]
- 12.Valerio M, et al. Evaluation of antifungal use in a tertiary care institution: Antifungal stewardship urgently needed. J. Antimicrob. Chemother. 2014;69:1993–1999. doi: 10.1093/jac/dku053. [DOI] [PubMed] [Google Scholar]
- 13.Valerio M, et al. Antifungal stewardship in a tertiary-care institution: A bedside intervention. Clin. Microbiol. Infect. 2015;21:492.e491–492.e499. doi: 10.1016/j.cmi.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Hamzavi SS, et al. Changing face of Candida colonization pattern in pediatric patients with hematological malignancy during repeated hospitalizations, results of a prospective observational study (2016–2017) in Shiraz, Iran. BMC Infect. Diseases. 2019;19:759. doi: 10.1186/s12879-019-4372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haddadi, P. et al. Yeast colonization and drug susceptibility pattern in the pediatric patients with neutropenia. Jundishapur J. Microbiol.7(9), e11858 (2014). [DOI] [PMC free article] [PubMed]
- 16.Mohammadi R, et al. Molecular identification and distribution profile of Candida species isolated from Iranian patients. Med. Mycol. 2013;51:657–663. doi: 10.3109/13693786.2013.770603. [DOI] [PubMed] [Google Scholar]
- 17.Lõoke M, Kristjuhan K, Kristjuhan A. Extraction of genomic DNA from yeasts for PCR-based applications. Biotechniques. 2011;50:325–328. doi: 10.2144/000113672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rex JH, Andes BD, Arthington-Skaggs B, Brown SD, Chaturvedi V, Ghannoum MA, Espinel-Ingroff A, Knapp CC, Ostrosky-Zeichner L, Pfaller MA, Sheehan DJ, Walsh TJ. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard. 3. Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 19.Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 4th ed. CLSI standard M27 (ISBN 1-56238-826-6 [Print]; ISBN 1-56238-827-4 [Electronic]). Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087 USA, (2017).
- 20.Arendrup MC, Kahlmeter G, Rodriguez-Tudela JL, Donnelly JP. Breakpoints for susceptibility testing should not divide wild-type distributions of important target species. Antimicrob. Agents Chemother. 2009;53:1628–1629. doi: 10.1128/AAC.01624-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnidge J, Kahlmeter G, Kronvall G. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin. Microbiol. Infect. 2006;12:418–425. doi: 10.1111/j.1469-0691.2006.01377.x. [DOI] [PubMed] [Google Scholar]
- 22.Gammelsrud K, et al. Colonization by Candida in children with cancer, children with cystic fibrosis, and healthy controls. Clin. Microbiol. Infect. 2011;17:1875–1881. doi: 10.1111/j.1469-0691.2011.03528.x. [DOI] [PubMed] [Google Scholar]
- 23.Alberth M, et al. Significance of oral Candida infections in children with cancer. Pathol. Oncol. Res. 2006;12:237. doi: 10.1007/BF02893420. [DOI] [PubMed] [Google Scholar]
- 24.Badiee P, et al. Antifungal susceptibility patterns of colonized Candida species isolates from immunocompromised pediatric patients in five university hospitals. Iran. J. Microbiol. 2017;9:363. [PMC free article] [PubMed] [Google Scholar]
- 25.Hovi L, Saarinen-Pihkala U, Vettenranta K, Saxen H. Invasive fungal infections in pediatric bone marrow transplant recipients: Single center experience of 10 years. Bone Marrow Transpl. 2000;26:999–1004. doi: 10.1038/sj.bmt.1702654. [DOI] [PubMed] [Google Scholar]
- 26.Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5:161–169. doi: 10.4161/viru.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sendid B, Lacroix C, Bougnoux M-E. Is Candida kefyr an emerging pathogen in patients with oncohematological diseases? Clin. Infect. Dis. 2006;43:666–667. doi: 10.1086/506573. [DOI] [PubMed] [Google Scholar]
- 28.Safdar A, Armstrong D. Prospective evaluation of Candida species colonization in hospitalized cancer patients: Impact on short-term survival in recipients of marrow transplantation and patients with hematological malignancies. Bone Marrow Transpl. 2002;30:931–935. doi: 10.1038/sj.bmt.1703732. [DOI] [PubMed] [Google Scholar]
- 29.Lau AF, et al. Candida colonization as a risk marker for invasive candidiasis in mixed medical-surgical intensive care units: Development and evaluation of a simple, standard protocol. J. Clin. Microbiol. 2015;53:1324–1330. doi: 10.1128/JCM.03239-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller, M. A. et al. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: Data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PloS One9(7), p.e101510 (2014). [DOI] [PMC free article] [PubMed]
- 31.Bougnoux M-E, et al. Multilocus sequence typing reveals intrafamilial transmission and microevolutions of Candida albicans isolates from the human digestive tract. J. Clin. Microbiol. 2006;44:1810–1820. doi: 10.1128/JCM.44.5.1810-1820.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alcazar-Fuoli L, Mellado E. Current status of antifungal resistance and its impact on clinical practice. Br. J. Haematol. 2014;166:471–484. doi: 10.1111/bjh.12896. [DOI] [PubMed] [Google Scholar]
- 33.Zareifar S, Badiee P, Haddadi P, Abdolkarimi B. Susceptibility pattern of anti-candida drugs in the pediatric patients with acute leukemia. Iran. J. Pediatr. Hematol. Oncol. 2017;7:1–8. [Google Scholar]
- 34.Organization, W.H. Antimicrobial Resistance: Global Report on Surveillance. World Health Organization; 2014. [Google Scholar]
- 35.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanguinetti M, Posteraro B, Lass-Flörl C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses. 2015;58:2–13. doi: 10.1111/myc.12330. [DOI] [PubMed] [Google Scholar]
- 37.Messer S, et al. Activities of micafungin against 315 invasive clinical isolates of fluconazole-resistant Candida spp. J. Clin. Microbiol. 2006;44:324–326. doi: 10.1128/JCM.44.2.324-326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niimi K, et al. Overexpression of Candida albicans CDR1, CDR2, or MDR1 does not produce significant changes in echinocandin susceptibility. Antimicrob. Agents Chemother. 2006;50:1148–1155. doi: 10.1128/AAC.50.4.1148-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cleveland AA, et al. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008–2013: results from population-based surveillance. PLoS ONE. 2015;10:e0120452. doi: 10.1371/journal.pone.0120452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanafani ZA, Perfect JR. Resistance to antifungal agents: Mechanisms and clinical impact. Clin. Infect. Dis. 2008;46:120–128. doi: 10.1086/524071. [DOI] [PubMed] [Google Scholar]
- 41.Tortorano AM, Prigitano A, Biraghi E, Viviani MA. The European Confederation of Medical Mycology (ECMM) survey of candidaemia in Italy: In vitro susceptibility of 375 Candida albicans isolates and biofilm production. J. Antimicrob. Chemother. 2005;56:777–779. doi: 10.1093/jac/dki310. [DOI] [PubMed] [Google Scholar]
- 42.Wilson LS, et al. The direct cost and incidence of systemic fungal infections. Value Health. 2002;5:26–34. doi: 10.1046/j.1524-4733.2002.51108.x. [DOI] [PubMed] [Google Scholar]
- 43.Hart E, Nguyen M, Allen M, Clark CM, Jacobs DM. A systematic review of the impact of antifungal stewardship interventions in the United States. Ann. Clin. Microbiol. Antimicrob. 2019;18:24. doi: 10.1186/s12941-019-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention . Antibiotic Resistance Threats in the United States. Department of Health and Human Services, CDC; 2019. [Google Scholar]
- 45.Maertens JA, Nucci M, Donnelly JP. The role of antifungal treatment in hematology. Haematologica. 2012;97:325. doi: 10.3324/haematol.2012.061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers, T. R. Defining Invasive Fungal Diseases for Clinical Research: A Work in Progress. 1377–1378 (2020). [DOI] [PubMed]
- 47.Warris A, et al. ESCMID-ECMM guideline: Diagnosis and management of invasive aspergillosis in neonates and children. Clin. Microbiol. Infect. 2019;25:1096–1113. doi: 10.1016/j.cmi.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Donnelly JP, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson MD, et al. Core recommendations for antifungal stewardship: A statement of the mycoses study group education and research consortium. J. Infect. Dis. 2020;222:S175–S198. doi: 10.1093/infdis/jiaa394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ullmann AJ, et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018;24:e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: Before and after chemoprophylaxis and institution of HEPA filters. Am. J. Hematol. 2001;66:257–262. doi: 10.1002/ajh.1054. [DOI] [PubMed] [Google Scholar]
- 52.Walsh TJ, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N. Engl. J. Med. 2002;346:225–234. doi: 10.1056/NEJM200201243460403. [DOI] [PubMed] [Google Scholar]
- 53.Lehrnbecher T, et al. Clinical practice guideline for systemic antifungal prophylaxis in pediatric patients with cancer and hematopoietic stem-cell transplantation recipients. J. Clin. Oncol. 2020;38:3205. doi: 10.1200/JCO.20.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Safdar A, Van Rhee F, Henslee-Downey J, Singhal S, Mehta J. Candida glabrata and Candida krusei fungemia after high-risk allogeneic marrow transplantation: No adverse effect of low-dose fluconazole prophylaxis on incidence and outcome. Bone Marrow Transpl. 2001;28:873–878. doi: 10.1038/sj.bmt.1703252. [DOI] [PubMed] [Google Scholar]
- 55.Sendid B, et al. Candidaemia and antifungal therapy in a French University Hospital: Rough trends over a decade and possible links. BMC Infect. Dis. 2006;6:80. doi: 10.1186/1471-2334-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beganovic M, Laplante KL. Communicating with facility leadership; metrics for successful antimicrobial stewardship programs (ASP) in acute care and long-term care facilities. R. I. Med. J. 2018;2013(101):45–49. [PubMed] [Google Scholar]
- 57.Dancer S, et al. Approaching zero: temporal effects of a restrictive antibiotic policy on hospital-acquired Clostridium difficile, extended-spectrum β-lactamase-producing coliforms and meticillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents. 2013;41:137–142. doi: 10.1016/j.ijantimicag.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Cook PP, Gooch M. Long-term effects of an antimicrobial stewardship programme at a tertiary-care teaching hospital. Int. J. Antimicrob. Agents. 2015;45:262–267. doi: 10.1016/j.ijantimicag.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Hwang H, Kim B. Impact of an infectious diseases specialist-led antimicrobial stewardship programmes on antibiotic use and antimicrobial resistance in a large Korean hospital. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-33201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rac H, Wagner JL, King ST, Barber KE, Stover KR. Impact of an antifungal stewardship intervention on optimization of candidemia management. Therap. Adv. Infect. Disease. 2018;5:3–10. doi: 10.1177/2049936117745267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rautemaa-Richardson R, et al. Impact of a diagnostics-driven antifungal stewardship programme in a UK tertiary referral teaching hospital. J. Antimicrob. Chemother. 2018;73:3488–3495. doi: 10.1093/jac/dky360. [DOI] [PubMed] [Google Scholar]
- 62.Apisarnthanarak A, Yatrasert A, Mundy LM. Impact of education and an antifungal stewardship program for candidiasis at a Thai tertiary care center. Infect. Control Hosp. Epidemiol. 2010;31:722–727. doi: 10.1086/653616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.