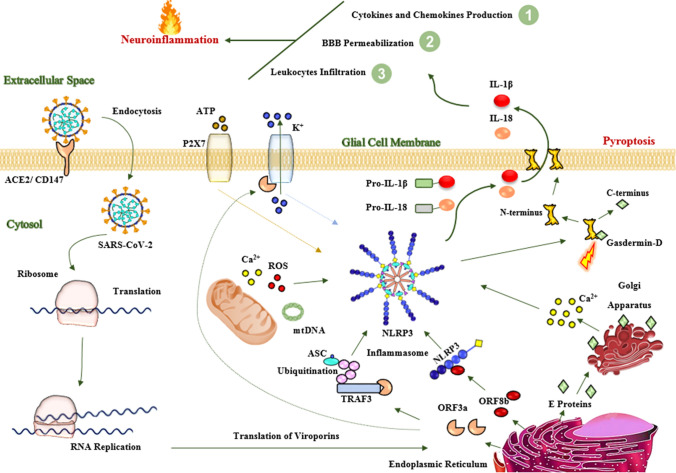
Fig. 3.
Proposed mechanisms of neuronal injury by SARS-CoV-2. In the CNS, SARS-CoV-2 enters into the glial cells, in particular microglia, through an endocytosis-dependent manner via interaction of spike proteins with ACE2 receptor. After internalization, viral RNA replicates and viral structural proteins, as well as viroporins, such as E protein, ORF3a, and ORF8b, are translated. The E proteins cause the release of calcium from the Golgi apparatus. The ORF8b interacts with NLRP3 protein. The ORF3a interacts with TRAF3 ubiquitinates ASC protein as well as increases the efflux of potassium from the cell membrane. These events along with mitochondrial dysfunctions, potassium efflux, and activated P2X7 receptors lead to activation of NLRP3 inflammasome and consequently activated caspase-1-induced pyroptosis in the glial cells. Activated proinflammatory cytokines, such as IL-1β and IL-18, trigger the production of other proinflammatory cytokines, such as TNFα, IFNβ, IL-6, and CCL2 into the CNS. IL-1β and IL-18 and other produced proinflammatory cytokines increase the permeability of the BBB and consequently enhance the infiltration of peripheral immune cells into the CNS. All these pathological processes cause severe neuroinflammation. Neuroinflammation is responsible for neuronal injury and subsequently neurological manifestations in SARS-CoV-2 infection. Abbreviations: ACE2 angiotensin-converting enzyme 2; ASC adaptor protein apoptosis-associated speck-like protein containing a caspase-recruitment domain; CD147 cluster of differentiation 147; E protein envelope protein; IL-1β interleukin 1beta; IL-18 interleukin18; mt DNA mitochondrial DNA; NLRP3 nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3; ORF3a open reading frame 3a; P2X7 purinergic type 2 ATP receptor family; ROS reactive oxygen species; TRAF3 tumor necrosis factor receptor‐associated factor 3