Abstract
Background:
Chronic obstructive pulmonary disease (COPD) is characterized by persistent respiratory symptoms and dyspnea, as well as an increase in the number of leukocytes in the airways, lungs, and pulmonary vessels. A ‘One size fits all’ approach to COPD patients with different clinical features may be considered outdated. The following are the two major objectives of this meta-analysis: the first is to determine if blood eosinophil counts (BEC) can serve as a prognostic biomarker of COPD outcomes, and the second is to determine which level of BEC is effective for inhaled corticosteroid (ICS) treatment.
Methods:
We searched articles published before 15 May 2021 in the following four electronic databases: Web of Science, Cochrane Library, EMBASE, and PubMed.
Results:
A total of 42 studies, comprising a sampling of 188,710 subjects, were summarized and compared in this meta-analysis. The rate ratio (RR) of exacerbations of COPD (ECOPD) between ICS and non-ICS treatment was statistically significant for the COPD patients with a baseline BEC ⩾ 2% or ⩾ 200 cells/μl, RR = 0.82 (0.73, 0.93) or 0.79 (0.70, 0.89) respectively, while the RR of ECOPD between ICS and non-ICS treatment was statistically insignificant for the COPD patients with baseline BEC < 2% or <200 cells/μl, RR = 0.97 (0.87, 1.08) or 0.97 (0.86, 1.08), suggested that ICS therapy was beneficial to the improvement of ECOPD in patients with a baseline BEC ⩾ 2% or BEC ⩾ 200 cells/μl.
Conclusion:
Our research shows that a BEC ⩾ 200 cells/μl or ⩾2% is likely to become the cutoff value of ICS treatment for ECOPD. Moreover, we believe that the baseline BEC can be used as a biomarker for predicting ECOPD. The stability of BEC requires special attention.
Keywords: biomarker, chronic obstructive pulmonary disease, eosinophil, exacerbations of COPD, inhaled corticosteroid
Introduction
Chronic obstructive pulmonary disease (COPD) is an inflammatory lung disease that affects 384 million people worldwide and causes 3.2 million deaths each year. 1 At present, the drug treatment for COPD is usually a combination of airway relaxants and inhaled anti-inflammatory drugs. The guidelines generally recommend a ‘one size fits all’ approach to COPD patients with different clinical features. However, despite the use of all the recommended therapies, some patients still have poor control of symptoms. In the era of personalized medical treatment, the simplicity of recommending only one treatment option in each situation of airway diseases may be considered outdated, and there is an urgent need for more targeted treatment strategy for patients.
Eosinophils (EOSs) develop and circulate briefly in the bone marrow, redistribute to the organs, including the thymus and gastrointestinal tract, and spread to the lungs to a lesser extent. 2 Transcription factors and cytokines, such as interleukin-3, interleukin-5, and granulocyte macrophage colony-stimulating factor, are critical for the differentiation of EOSs, while interleukin-5 plays a key role in their maturation, recruitment, and activation at the inflammatory site. Currently, studies have shown that EOSs lead to airway inflammation and bronchial hyperresponsiveness and are important cells leading to asthma. However, the role of EOSs in COPD remains unclear. Some studies reported that the risk of exacerbations of COPD (ECOPD) increased with the change of blood EOS counts (BECs) in the general and clinical populations,3–5 as well as in post hoc analysis of clinical trials;6–9 while others reported that there was a lack of correlation between BEC and ECOPD.10–14 Recently, a clinical trial revealed that mepolizumab, an anti–IL-5 monoclonal antibody (mAb), could reduce the moderate or severe exacerbation of COPD patients with high BEC, 15 indicating that BEC was a useful biomarker for the identification of eosinophilic inflammation that can be targeted for therapy. Whether BEC can be a biomarker to predict ECOPD patients and the exact BEC threshold before inhaled corticosteroids (ICSs) have an effect is debated.
The following are the two major objectives of this meta-analysis: the first is to determine if BEC can serve as a prognostic biomarker of COPD outcomes, and the second is to determine which level of BEC is effective for ICS treatment.
Methods
Protocol and guidance
The guidelines used in this review and meta-analysis are the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA). 16
Eligibility criteria
In this study, randomized controlled trials (RCTs) and post hoc analyses of RCTs were included to evaluate whether BEC was a marker of the response of patients with COPD to ICS. The patients in the included studies were all patients with COPD divided into two groups according to the cutoff point of BEC, that is, above and below the cutoff point. Patients with asthma/allergy were excluded. The intervention measures in all studies were treatment with ICS, while the controls were not treated with ICS. The outcome of all studies was the change in forced expiratory volume in 1 s (FEV1) from baseline, Saint George’s Respiratory Questionnaire (SGRQ) total score change from baseline, or the rate ratio (RR) of ECOPD.
The observational cohort studies were included to evaluate whether the BEC is a prognostic biomarker in patients with COPD. The patients in the included studies were all patients with COPD divided into two groups according to the cutoff point of BEC, that is, above and below the cutoff point. The outcome of all studies was the RR of prognostic ECOPD or the all-cause mortality hazard ratio (HR).
Information sources and search strategy
We searched articles published before 15 May 2021 in the following four electronic databases: Web of Science, Cochrane Library, EMBASE, and PubMed. These articles were manually screened, regardless of the language or data, using the following search terms: ((biomarker [Title/Abstract]) OR (marker [Title/Abstract])) AND (eosinophil) AND ((COPD[Title/Abstract]) OR (chronic obstructive pulmonary disease [Title/Abstract]) OR chronic obstructive pulmonary disease [MeSH Terms]).
Study selection
The titles and abstracts of the studies were independently screened by two methodologically competent reviewers to determine whether the cited articles met the eligibility criteria. Only after they reached an agreement over differences through consensus discussion or arbitration by a third reviewer could they read the full text and extract relevant data. The reasons for inclusion or exclusion were documented in detail. Case reports, letters, and minutes of meetings were not included. The PRISMA flowchart was used to summarize the study selection processes.
Data extraction
Two investigators initially used a predefined data extraction sheet to independently perform data extraction from each included study, such as sputum or BEC cut point, primary endpoint, counts and effect estimates, country, follow up years, title, conclusion, and other data, including study design, grouping and number of people in the group, sample size, authors, publication year, population, age, and male%. The third investigator independently verified the data to ensure accuracy. If no data were available in digital format, we estimated data from the graphs using the free software Plot Digitizer.
Definition of outcomes
Primary outcomes
The difference in the mean change in FEV1 between ICS therapy and non-ICS therapy.
The difference in the mean change in SGRQ score between ICS therapy and non-ICS therapy. 17
-
The RR of ECOPD between ICS therapy and non-ICS therapy.
Remark: Each of the three outcomes above has the following two pooled effect values: one is for COPD patients whose BEC is above the cutoff point and the other is below the cutoff point.
The above three outcomes were used to determine whether ICS is a useful treatment for patients with COPD and high BEC.
The RR of prognostic ECOPD between patients with baseline BEC above and below the cutoff point (cutoff point = 2%, 3%, 4%, 5%, 150, 200, 300, 400, and 500 cells/μl).
-
The HR of all-cause mortality between patients with a baseline BEC ⩾ cutoff point and a BEC < cutoff point.
The above two outcomes were used to determine whether BEC can serve as a prognostic biomarker of COPD outcomes.
Secondary outcomes
The mean difference in baseline FEV1/FVC and the odds ratio (OR) of the baseline GOLD III + IV between a BEC ⩾ 2% and a BEC < 2% among patients with COPD.
Statistical analysis
The random effects model and inverse variance method were used to summarize the effect size assuming heterogeneity always existed. We reported the pooled estimates as the weighted mean difference along with their respective 95% confidence interval (CI). Cross-study heterogeneity was assessed using the Cochran Q test, and a p-value < 0.10 was considered significant. We also calculated the I² statistic as a measure of cross-study inconsistency, and statistical heterogeneity was considered significant when the I² index > 50%. This meta-analysis was performed using RevMan v5.3 (Cochrane Collaboration, Copenhagen, Denmark). We originally intended to assess publication bias by using visual inspection of funnel plots and the Egger regression asymmetry test. We were unable to conduct a formal test because there were fewer than 10 studies available for comparisons.
Assessment of risk of bias in individual studies
The qualities of RCTs were assessed using the Cochrane Handbook for Systematic Reviews of Interventions. We assessed the risk of bias for the following domains: selection (random sequence generation, allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome); attrition (incomplete outcome data); reporting (selective reporting); and other unclear bias. 18 To assess the risk of bias of observational studies, we followed the Newcastle-Ottawa Quality Assessment Scale. The NOS statement was judged on three broad perspectives (selection, comparability, and outcome) consisting of eight items. 19
Additional analysis
In addition to the cutoff point of BEC 2%, we planned to analyze multiple cutoff points of BEC (3%, 4%, and 5%, and 150, 200, 300, 400, and 500 cells/μl).
Results
Study selection
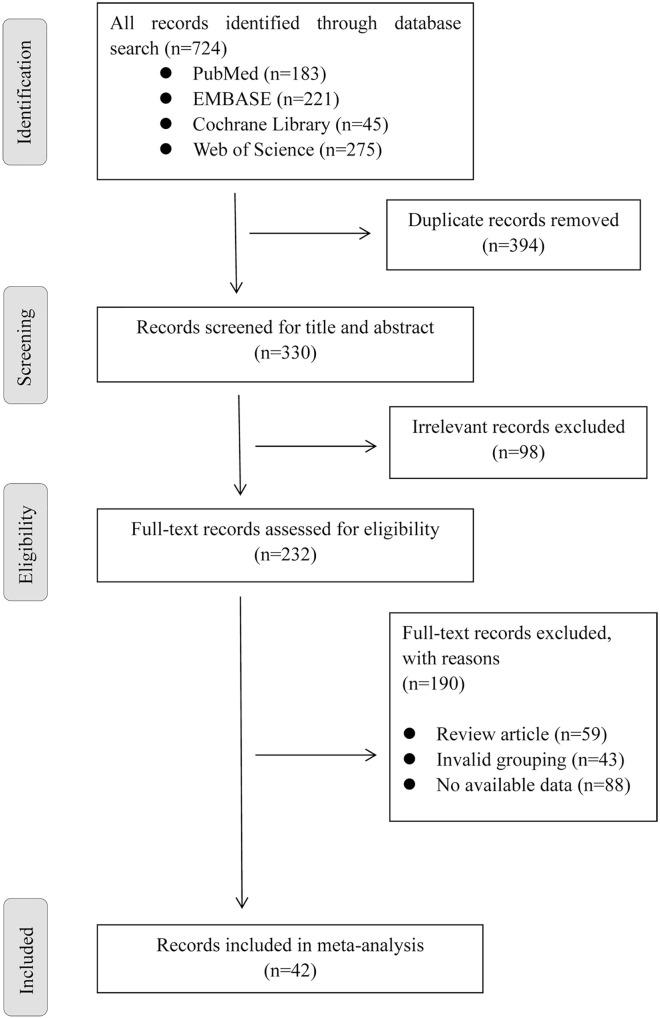
The initial search of four databases yielded 724 publications. After reading the title and abstract and excluding duplicate and irrelevant articles, we obtained 232 articles. After manually reading the full text, 157 articles were excluded, including review articles (n = 59), invalid grouping (n = 43), and no available data (n = 88). In the end, 42 articles were included in the meta-analysis (Figure 1).
Figure 1.
Flowchart of study selection.
Study characteristics and risk of bias within studies
A total of 42 studies, comprising a sampling of 188,710 subjects, were summarized and compared in this meta-analysis. Among them, 13 RCTs (three RCTs from the same study Pavord et al. 20 ) were used to assess whether BEC was a marker of response to ICS in COPD patients. All 13 RCTs were double-blind, ten of which did not use placebo, and the randomization was described. Since Singh 2020 pooled data from 11 clinical trials, there may be a high risk of selection bias and reporting bias. We know that continued smoking is associated with an impaired response to ICS and thereby affects the attainment of important clinical outcomes in COPD patients; therefore, in all RCTs included in this study, smoking status was balanced between groups. We used 29 cohort studies to analyze the relationship between BEC and the clinical characteristics of patients with COPD and whether the level of BEC was a marker for the prognosis of COPD patients. Most of these cohort studies obtained records from electronic healthcare records, and there were no studies with low quality, as evaluated from patient selection, comparability, exposure assessment, or outcome assessment. The mean age of the population in all studies was greater than 60 years, of which 16 studies had a mean age greater than 70 years. The detailed information of each study is listed in Table 1. Risk-of-bias assessments of RCTs are reported in Supplemental Figures S1 and S2, and risk-of-bias assessments of observational studies are reported in Supplemental Table S1.
Table 1.
Characteristics of included studies.
| Study | Study design | Age | Male % | Sample size | Cut point | Country | Follow-up |
|---|---|---|---|---|---|---|---|
| Aksoy et al. 21 | Observational cohort | 67 (59–75) | 72.0 | 10,593 | 2% | Turkey | 0 |
| Bafadhel et al. 22 | Observational cohort | 72 (48–89) | 47.7 | 243 | ⩾200 cells/μl and/or ⩾2% | UK | 1 year |
| Bafadhel et al. 23 | RCT double-blind | 70 (49–87) | 55.0 | 109 | 2% | UK | 6 weeks |
| Barnes et al. 9 ISOLDE 9 | RCT double-blind | 63.5 ± 7.34 | 82.0 | 738 | 2% | UK | 3 years |
| Bélanger et al. 24 | Observational cohort | 68.7 ± 9.4 | 56.6 | 479 | ⩾200 cells/ml and ⩾2% | Canada | 1 year |
| Chan et al. 25 | Observational cohort | 74.2 ± 8.3 | 91.5 | 247 | 2% | Hong Kong | 1 year |
| Chapman et al. 26 | RCT | 65.3 ± 7.80 | 70.6 | 743 | 2% 150, 300 cells/μl | UK | 26-weeks |
| Cheng and Lin 27 | Observational cohort | 68.7 ± 19.2 | 81.2 | 248 | 3% | Taiwan | 0 |
| Couillard et al. 28 | Observational cohort | 69.3 ± 11.0 | 50.9 | 167 | 2% | USA | 1 year |
| Disantostefano et al. 29 | Observational study | – | 59.7 | 4071 | 2% | USA | 0 |
| Duman et al. 30 | Observational cohort | 70 (61–80) | 66.9 | 1704 | 2% | Turkey | 6 months |
| Ferguson et al. 31 | RCT | 64.9 (7.8) | 72.0 | 1902 | 150 cells/μl | USA | 24 weeks |
| Gonzalez-Barcala et al. 32 | Observational cohort | 74.34 (11.1) | 77.1 | 358 | 200, 300, 400 cells/μl | Spain | 30 days |
| Hasegawa and Camargo 33 | Observational cohort | 71 (62–79) | 57.0 | 3084 | 300 cells/μl | USA | 1 year |
| Hastie et al. 14 | Prospective cohort (SPIROMICS) | 65 (59–71) | 59.0 | 2499 | 200/μl | USA | 1 year |
| Hegewald et al. 34 | Observational cohort | 68.4 ± 11.6 | 50.7 | 2445 | 70, 220, 300, 400, 500 cells/μl | USA | 1 year |
| Kerkhof et al. 4 | Observational cohort | 70 ± 10 | 56.0 | 8318 | UK | >1 year | |
| Landis et al. 35 | Observational cohort | 71.1 (10.6) | 51.5 | 55114 | UK | 1 year | |
| Lv et al. 36 | Observational cohort | 65.69 ± 9.96 | 55.7 | 174 | 2%, 4% | China | 0 |
| Mendy et al. 37 | Observational cohort | – | 75.1 | 431 | 2% | USA | 36 months |
| Nishimura et al. 38 | Observational cohort | 74.9 ± 6.7 | 91.1 | 135 | 100, 300 cells/μl | Japan | 0 |
| Oh et al. 39 | Prospective cohort | 66.9 ± 7.5 | 97.5 | 629 | High (⩾5%), middle (2–5%), low (<2%). | South Korea | 2.2 years ± 1.8 |
| Oshagbemi et al. 40 | Observational cohort | 64.8 (10.8) | 55.5 | 32,693 | 2%, 4%, 6%, 340 cells/μl | Netherlands | 3 years |
| Papi et al. 41 | RCT | 63.8 (7.92) | 75.5 | 1765 | 2%, 3%, 4% | Italy | 52 weeks |
| Papi et al. 42 | RCT | 64.4 (7.7) | 72.0 | 1532 | 200 cells/μl, 2% | Italy | 52 weeks |
| Pascoe et al. 6 | A secondary analysis of data from two double-blind RCTs | 63.8 (9.2) | 59.0 | 3177 | 2% | USA | 1 year |
| Pavord et al. 20 (SCO30002) | RCT double-blind | 64.4 (9.08) | 87.0 | 373 | 2% | UK | 1 year |
| Pavord et al. 20 (SCO40036) | RCT double-blind | 64.3 (8.06) | 82.0 | 1269 | 2% | UK | 1 year |
| Pavord et al. 20 (SFCB3024) | RCT double-blind | 63.1 (8.49) | 77.0 | 1403 | 2% | UK | 1 year |
| Peng et al. 43 | Observational cohort | 71.1 ± 9.6 | 73.2 | 123 | 200, 300, 400 cells/μl | China | 12-month |
| Poder et al. 44 | Observational cohort | 68.9 ± 9.4 | 52.0 | 479 | ⩾200 cells/Ml and/or ⩾2% | Canada | 1 year |
| Prins et al. 45 | Observational cohort | 70.4 (8.7) | 59.0 | 207 | 2%, 300 cells/μl | Netherlands | 180 days |
| Roche et al. 46 | Observational cohort | 64.8 (7.73) | 77.8 | 3079 | (2%, 3% and ⩾3%, 5%) 150, 300 cells/μl | France | 1 year |
| Serafino-Agrusa et al. 47 | Case control | 72.9 ± 8.6 | 90.0 | 132 | 2% | Italy | 2 years |
| Siddiqui et al. 7 | A secondary analysis of data from double-blind RCT | 63.6 (8.3) | 73.6 | 1184 | 110.4, 181.6, 279.8 | UK | 48 weeks |
| Singh et al. 48 | RCT | 65.1 (8.6) | 74.1 | 22,125 | 150 300 cells/μl | UK | Minimum of 48 weeks |
| Song et al. 49 | Prospective cohort | 69.5 ± 7.4 | 95.9 | 467 | 200, 300, 400, 500, 600 cells/μl | Republic of Korea | 1 year |
| Vedel-Krogh et al. 50 | Prospective study | 71 (66–78) | 75.0 | 7180 | ⩾340 cells/μl | Denmark | 3.7 years |
| Vestbo et al. 51 | RCT | 63.4 (8.7) | 77.0 | 2691 | 200 cells/μl, 2% | Italy | 52 weeks |
| Watz et al. 8 | A secondary analysis of data from double-blind, parallel-group RCT | 64.1 (8.6) | 82.0 | 2420 | 2%, 4% | Germany | 1 year |
| Yun et al. 52 (COPDGene) | Observational cohort | 68.27 (8.29) | 64.5 | 1553 | 300 cells/ml | USA | 3 years |
| Yun et al. 52 (ECLIPSE) | Observational cohort | 63.86 (6.8) | 72.7 | 1895 | 300 cells/ml | USA | 3 years |
| Zeiger et al. 5 | Observational cohort | 71.5 (9.6) | 57.1 | 7245 | 50 150 300 400 500 | USA | 1 year |
| Zhang et al. 53 | Observational cohort | 90% > 60 | 70.9 | 829 | 150, 200, 300 cells/μl | China | 46 months (33–54) |
| Zysman et al. 10 | Observational cohort | 62 (55–70) | 72.6 | 458 | 2% 3% 4% | France | 48 months |
| Total studies | 42 | 188,710 | |||||
Synthesis of results
Primary outcomes
FEV1: mean change from baseline; RCT, randomized control trial
Relative BEC: First, the mean difference in the change in FEV1 was significant between ICS and placebo users or between ICS and non-ICS users in COPD patients with a baseline BEC ⩾ 2% [mean difference (MD) = 38.76 (20.18, 57.34) and 32.03 (1.95, 62.11), respectively]. In contrast, a significant mean difference change in FEV1 was not found in COPD patients with a baseline BEC < 2%. The results showed that the effect of ICS on FEV1 was significantly better than that of placebo or non-ICS in COPD patients with a baseline BEC ⩾ 2%. Second, a mean difference change in FEV1 was not found between ICS + long-acting b2-agonist (LABA)/long-acting muscarinic antagonist (LAMA) and LABA/LAMA users, regardless of whether the COPD patients had a baseline BEC ⩾ 2% or <2% (Table 2).
Table 2.
FEV1 mean change and SGRQ score change from baseline.
| Outcome | Comparisons | Effect size (BEC < 2%) | Effect size (BEC ⩾ 2%) | Studies included |
|---|---|---|---|---|
| MD of FEV1 change | ICS versus non-ICS | 2.36 (−26.23, 30.95) | 30.71 (−0.11, 61.52) | 9 |
| ICS versus placebo | 23.56 (−24.64, 71.77) | 38.76 (20.18, 57.34) | 4 | |
| ICS+LAMA/LABA versus LAMA/LABA | 0.82 (−42.17, 43.82) | 27.15 (−13.10, 67.39) | 6 | |
| ICS versus non-ICS | Effect size (BEC < 150 cells/μl) | Effect size (BEC ⩾150 cells/μl) | 3 | |
| −22.33 (−64.20, 19.54) | 8.33 (−66.82, 83.49) | |||
| Effect size (BEC < 300 cells/μl) | Effect size (BEC ⩾ 300 cells/μl) | |||
| 0.52 (−63.72, 64.77) | 38.16 (−63.44, 139.77) | |||
| MD of SGRQ score change | ICS versus non-ICS | −1.30 (−4.05, 1.45) | −1.12 (−2.60, 0.35) | 6 |
| ICS versus placebo | −1.97 (−6.62, 2.68) | −2.85 (−7.95, 2.26) | 2 | |
| ICS + LAMA/LABA versus LAMA/LABA | −0.62 (−3.35, 2.11) | −0.32 (−1.29, 0.65) | 5 |
BEC, blood eosinophil counts; FEV1, forced expiratory volume in one second; ICS, inhaled corticosteroid; LABA, long-acting b2-agonist; LAMA, long-acting muscarinic antagonist; MD, mean difference; SGRQ: Saint George’s Respiratory Questionnaire.
Absolute BEC: We analyzed the thresholds of 150 and 300 cells/μl, and there was no difference in FEV1 mean changes between ICS and non-ICS therapy, whether in the group below the threshold or above the threshold (Table 2). The results suggested that dividing patients by a cutoff point of 150 or 300 cells/μl could not distinguish whether ICS was beneficial to the improvement of FEV1 (Table 2).
SGRQ: mean change from baseline
A mean difference change in SGRQ was not found between ICS and placebo users, between ICS and non-ICS users, or between ICS+LABA/LAMA and LABA/LAMA users, regardless of whether the COPD patients had a baseline BEC ⩾ 2% or <2% (Table 2).
The RR of ECOPD between ICS therapy and non-ICS therapy
Relative BEC: First, the RR of ECOPD between ICS and non-ICS treatment was statistically insignificant, regardless of whether the COPD patients had a baseline BEC ⩾ 3% or <3% [RR = 0.83 (0.66, 1.04) and 0.98 (0.86, 1.12), respectively]. The results suggested that dividing patients by a cutoff point of 3% could not distinguish whether ICS was beneficial to the improvement of ECOPD. Second, the RR of ECOPD between ICS and non-ICS treatment was statistically significant for COPD patients with a baseline BEC ⩾ 2% [RR = 0.82 (0.73, 0.93)], while the RR of ECOPD between ICS and non-ICS treatment was statistically insignificant for COPD patients with a baseline BEC < 2% [RR = 0.97 (0.87, 1.08)]. The results suggested that ICS therapy was beneficial to the improvement of ECOPD in patients with a baseline BEC ⩾ 2%, but it showed no difference in COPD patients with a baseline BEC < 2% (Table 3).
Table 3.
Rate ratio of the exacerbations of COPD between ICS therapy and non-ICS therapy in patients with baseline BEC ⩾ cutoff points and BEC < cutoff points.
| Cutoff points | ⩾Cutoff points | <Cutoff points |
|---|---|---|
| 2% | 0.82 (0.73, 0.93) | 0.97 (0.87, 1.08) |
| 3% | 0.83 (0.66, 1.04) | 0.98 (0.86, 1.12) |
| 150 cells/μl | 0.79 (0.62, 1.01) | 0.93 (0.77, 1.11) |
| 200 cells/μl | 0.79 (0.70, 0.89) | 0.97 (0.86, 1.08) |
| 300 cells/μl | 0.76 (0.48, 1.21) | 1.06 (0.92, 1.23) |
BEC, blood eosinophil counts; COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid.
Absolute BEC: We analyzed the thresholds of 150, 200 and 300 cells/μl. Only the RR of ECOPD between ICS and non-ICS treatment was statistically significant for the COPD patients with a baseline BEC ⩾ 200 cells/μl [RR = 0.79 (0.70, 0.89)], while for the thresholds of 150 and 300 cells/μl, the RR of ECOPD between ICS and non-ICS treatments was not statistically significant for COPD patients regardless of whether baseline BEC was greater than or less than the threshold. The results suggested that ICS therapy was beneficial to the improvement of ECOPD in patients with a baseline BEC ⩾ 200 cells/μl (Table 3).
RR of ECOPD between patients with baseline BEC higher than thresholds versus baseline BEC lower than thresholds
Relative BEC: The pooled RRs of ECOPD between patients with a baseline BEC above 2%, 3%, 4%, and 5% and below 2%, 3%, 4%, and 5% suggested that patients with a baseline BEC above the cutoff points always had a significantly (except 2% which was no difference) higher exacerbation rate of COPD during 6 months to 3-years follow-up than their counterparts with a baseline BEC below the cutoff points, and the higher the cutoff point, the bigger the RR (Table 4).
Table 4.
Rate ratio of ECOPD in patients with baseline BEC higher than cutoff point versus BEC lower than cutoff point.
| Item | Cutoff points | Effect size | Studies included |
|---|---|---|---|
| ECOPD | BEC ⩾ 2% | 1.19 (0.82, 1.72) | 11 |
| BEC ⩾ 3% | 1.38 (1.15, 1.66) | 9 | |
| BEC ⩾ 4% | 1.52 (1.30, 1.77) | 4 | |
| BEC ⩾ 5% | 1.75 (1.47, 2.09) | 3 | |
| BEC ⩾ 150 cells/μl | 1.06 (1.00, 1.12) | 3 | |
| BEC ⩾ 200 cells/μl | 1.42 (1.10, 1.85) | 6 | |
| BEC ⩾ 300 cells/μl | 1.24 (1.14, 1.35) | 12 | |
| BEC ⩾ 400 cells/μl | 1.51 (1.31, 1.75) | 7 | |
| BEC ⩾ 500 cells/μl | 1.77 (1.46, 2.14) | 2 | |
| Survival | BEC ⩾ 2% | 0.85 (0.57, 1.24) | 4 |
| BEC ⩾ 200 cells/μl | 0.80 (0.62, 1.02) | 3 | |
| BEC ⩾ 300 cells/μl | 0.81 (0.71, 0.93) | 5 |
BEC, blood eosinophil count; COPD, chronic obstructive pulmonary disease; ECOPD, exacerbations of COPD.
Absolute BEC: The pooled RRs of ECOPD between patients with a baseline BEC above 150, 200, 300, 400, and 500 cells/μl and below 150, 200, 300, 400, and 500 cells/μl suggested that patients with baseline BECs above the thresholds always had a significantly higher exacerbation rate of COPD during the 6-month to 3-year follow-up than their counterparts with baseline BECs below the thresholds, and the risk of ECOPD showed an upward trend with the increase of the thresholds (Table 4).
HR of all-cause mortality in patients with baseline BEC ⩾ thresholds versus BEC < thresholds
Relative BEC: The HR of all-cause mortality between patients with a baseline BEC ⩾ 2% and < 2% was 0.85 (0.57, 1.24), indicating that a difference was not found between patients with a baseline BEC ⩾ 2% and <2% in the prognosis of all-cause mortality during the 6-month to 3-year follow-up (Table 4).
Absolute BEC: The HR of all-cause mortality between patients with a baseline BEC ⩾ 200 and <200 cells/μl was 0.80 (0.62, 1.02), and the HR of all-cause mortality was 0.81 (0.71, 0.93) for the 300 cells/μl threshold, suggesting that the difference was significant between patients with a baseline BEC ⩾ 300 cells/μl and <300 cells/μl in the prognosis of all-cause mortality during the 6-month to 3-year follow-up (Table 4).
Secondary outcomes
The mean difference in the pooled baseline FEV/FVC was statistically insignificant between COPD patients with a baseline BEC ⩾ 2% and <2% [MD = 0.85 (−0.26, 1.96)], while the OR of the pooled GOLD⩾III was statistically insignificant between the COPD patients with a higher baseline BEC and those with a lower baseline BEC [OR = 0.98 (0.89, 1.08)], suggesting that the BEC level cannot distinguish the severity of the disease (Table 5).
Table 5.
Secondary outcomes.
| Outcome | Effect size | Studies included |
|---|---|---|
| OR of GOLD III+IV in patients with baseline high BEC versus low BEC | 0.98 (0.89, 1.08) | 9 |
| MD of baseline FEV1/FVC in patients with baseline BEC ⩾ 2% versus BEC < 2% | 0.85 (−0.26, 1.96) | 7 |
BEC, blood eosinophil counts; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; MD, mean difference; OR, odds ratio.
Each effect size in Table 2–5 corresponds to a Forest plot. Please refer to the Supplemental Figures S3–S42.
Discussion
A total of 42 studies involving 188,710 patients were included in this study. We discussed the possibility of using BEC as a biomarker for COPD in the following two ways: the first was to assess whether BEC could serve as a prognostic biomarker of COPD outcomes and the second was to assess whether inhaled corticosteroid (ICSs) are a useful treatment for patients with COPD and a high BEC.
BEC threshold to guide ICS treatment
The main goal of COPD patient management is to prevent disease exacerbation. The general treatment for ECOPD is the use of bronchodilators, and the effect of ICS is uncertain. However, the main treatment for asthma is inhaled corticosteroids. Almost all asthma patients can benefit from ICS. 54 If RCTs do not exclude asthma patients, the response to ICS will be overestimated. Therefore, all RCTs included in this study excluded patients with asthma. In patients with COPD, the individual patient response is uncertain, and there is concern about the potential for serious side effects of ICS therapy, such as an increased risk of pneumonia.55–58 Therefore, current COPD guidelines recommend that treatment should be based not only on the degree of pulmonary impairment but also on the presence of other risk factors. 59 BEC is an easily accessible and interpretable indicator that may be suitable for use as a biomarker to identify which patients are most likely to benefit from inhaled corticosteroids. 6 Many opinions currently hold that ⩾2% is likely to be a relatively appropriate cutoff point for determining treatment efficacy with ICS.6,20,60 Oshagemi et al. 61 found an overall reduction in the risk of moderate or severe exacerbations in patients with an absolute BEC ranging from ⩾100 to ⩾340 cells/μl. Harries et al. 62 found three blood eosinophil thresholds of 2%, 150 cells/μl and 300 cells/μl. Our meta-analysis explored more BEC thresholds (including relative BEC of 2% and 3% and an absolute BEC of 150, 200, and 300 cells/μl) to identify the exact phenotypes of COPD that benefit from ICS. We confirmed 2% and 200 cells/μl and rejected 150 and 300 cells/μl and 3%. We found that among COPD patients with a BEC ⩾ 2%, patients treated with ICS had a 17% lower risk of ECOPD than patients not treated with ICS, while no difference was found in COPD patients with a BEC < 2%. Regardless of having a BEC ⩾ 3% or <3%, there was no difference in the risk of ECOPD in COPD patients with ICS compared with non-ICS treatment. In addition, we found that among COPD patients with a BEC ⩾ 200 cells/μl, patients treated with ICS had a 21% lower risk of ECOPD than patients not treated with ICS, while no difference was found in COPD patients with a BEC < 200 cells/μl. At BEC thresholds of 150 and 300 cells/μl, there was no difference in the risk of ECOPD in COPD patients with ICS compared with non-ICS treatment. We found that 2% and 200 cells/μl were expected to be the thresholds for guiding ICS.
It is worth noting that the association between EOS and ICS may be more complex than has been anticipated thus far. A study suggested that ICS may affect EOS levels, so the resting EOS threshold to guide ICS treatment of ECOPD may not be appropriate for every individual. The degree of change in EOS after ICS treatment might be a more accurate predictor of whether ICS benefits ECOPD. 63 More clinical trials may be needed to confirm this.
BEC is a potential biomarker of ECOPD
COPD patients are susceptible to periodic deterioration of their disease, which is mainly caused by bacterial and viral pathogens, known as ECOPD. Frequent ECOPD can accelerate lung function decline and has a significant impact on quality of life, morbidity and mortality. 64 Therefore, it is generally believed that a biomarker is needed to predict ECOPD. A recent study by Bafadhel et al. 65 showed that BEC predicted the risk of exacerbations. However, it remains controversial to choose a suitable cutoff point. Observational cohort studies were included in our study to evaluate which BEC threshold (150, 200, 300, 400, or 500 cells/μl, 2%, 3%, 4%, or 5%) was a prognostic survival and exacerbation biomarker. We found that in the real world, COPD patients with a baseline BEC above the threshold always have a significantly higher risk of ECOPD than patients with a baseline BEC below the threshold during a follow-up period of 6 months to 3 years, and the risk ratio increased as the threshold increased. However, this trend did not apply to survival and seemed to be the opposite. Since there are few studies on the association of BEC and survival, this result needs to be interpreted with caution.
Limitations
First, a clear explanation of the mechanism by which BEC pathways regulate the response to ICS and their impact on the progression of the disease is currently lacking. Second, as a biomarker for predicting ECOPD, the stability of BEC is particularly important. Studies have shown that age and sex will affect BEC stability.66,67 However, the articles included in this study were all based on the BEC at the baseline timepoint, without considering the stability of BEC over a considerable period of time.
Conclusions
In summary, our research shows that a BEC ⩾ 200 cells/μl or ⩾2% is likely to become the cutoff value of ICS treatment for ECOPD. Moreover, we believe that the baseline BEC can be used as a biomarker for predicting ECOPD. The stability of BEC requires special attention. COPD is a multiphenotypic disease with complex causes. We hope to make a contribution to the precise treatment of COPD through our research on the BEC threshold.
Supplemental Material
Supplemental material, sj-pdf-1-taj-10.1177_20406223211028768 for Blood eosinophil count-guided corticosteroid therapy and as a prognostic biomarker of exacerbations of chronic obstructive pulmonary disease: a systematic review and meta-analysis by Tao Liu, Zi-Jian Xiang, Xiao-Meng Hou, Jing-Jing Chai, Yan-Li Yang and Xiao-Tong Zhang in Therapeutic Advances in Chronic Disease
Footnotes
Abbreviations: BEC, Blood Eosinophil counts; CI, Confidence Interval; COPD, Chronic Obstructive Pulmonary Disease; ECOPD, Exacerbations of COPD; EOS, Eosinophil; FEV1, Forced Expiratory Volume in One Second; FVC, Forced Vital Capacity; HR, Hazard Ratio; ICS, Inhaled Corticosteroid; LABA, Long-acting b2-agonist; LAMA, Long-acting Muscarinic Antagonist; MD, Mean Difference; OR, Odds Ratio; RCT, Randomized Controlled Trial; RR, Rate Ratio; SGRQ: Saint George’s Respiratory Questionnaire.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (No. 2018-12M-1-003).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Xiao-Tong Zhang  https://orcid.org/0000-0001-8937-820X
https://orcid.org/0000-0001-8937-820X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Tao Liu, Department of Pulmonary and Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Zi-Jian Xiang, Beijing Zhiyun Data Technology Co. LTD, Beijing, China.
Xiao-Meng Hou, Department of Health Care, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Jing-Jing Chai, Department of Emergency Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Yan-Li Yang, Department of Pulmonary and Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Xiao-Tong Zhang, Department of Pulmonary and Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No.1 Shuaifuyuan Wangfujing Dongcheng District, Beijing, 100730, China.
References
- 1. Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health 2015; 5: 020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol 2009; 101: 81–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vedel-Krogh S, Nielsen SF, Lange P, et al. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen general population study. Am J Respir Crit Care Med 2016; 193: 965–974. [DOI] [PubMed] [Google Scholar]
- 4. Kerkhof M, Sonnappa S, Postma DS, et al. Blood eosinophil count and exacerbation risk in patients with COPD. Eur Respir J 2017; 50: 1700761. [DOI] [PubMed] [Google Scholar]
- 5. Zeiger RS, Tran TN, Butler RK, et al. Relationship of blood eosinophil count to exacerbations in chronic obstructive pulmonary disease. J Allergy Clin Immunol Pract 2018; 6: 944–954.e945. [DOI] [PubMed] [Google Scholar]
- 6. Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015; 3: 435–442. [DOI] [PubMed] [Google Scholar]
- 7. Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watz H, Tetzlaff K, Wouters EF, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med 2016; 4: 390–398. [DOI] [PubMed] [Google Scholar]
- 9. Barnes NC, Sharma R, Lettis S, et al. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J 2016; 47: 1374–1382. [DOI] [PubMed] [Google Scholar]
- 10. Zysman M, Deslee G, Caillaud D, et al. Relationship between blood eosinophils, clinical characteristics, and mortality in patients with COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 1819–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turato G, Semenzato U, Bazzan E, et al. Blood eosinophilia neither reflects tissue eosinophils nor worsens clinical outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 197: 1216–1219. [DOI] [PubMed] [Google Scholar]
- 12. Kim VL, Coombs NA, Staples KJ, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J 2017; 50: 1700853. [DOI] [PubMed] [Google Scholar]
- 13. Casanova C, Celli BR, de-Torres JP, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J 2017; 50: 1701162. [DOI] [PubMed] [Google Scholar]
- 14. Hastie AT, Martinez FJ, Curtis JL, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med 2017; 5: 956–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med 2017; 377: 1613–1629. [DOI] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis 1992; 145: 1321–1327. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of case-control studies in meta-analyses. Eur J Epidemiol 2011; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 20. Pavord ID, Lettis S, Locantore N, et al. Blood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPD. Thorax 2016; 71: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aksoy E, Karakurt Z, Gungor S, et al. Neutrophil to lymphocyte ratio is a better indicator of COPD exacerbation severity in neutrophilic endotypes than eosinophilic endotypes. Int J Chron Obstruct Pulmon Dis 2018; 13: 2721–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bafadhel M, Greening NJ, Harvey-Dunstan TC, et al. Blood eosinophils and outcomes in severe hospitalized exacerbations of COPD. Chest 2016; 150: 320–328. [DOI] [PubMed] [Google Scholar]
- 23. Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2012; 186: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bélanger M, Couillard S, Courteau J, et al. Eosinophil counts in first COPD hospitalizations: a comparison of health service utilization. Int J Chron Obstruct Pulmon Dis 2018; 13: 3045–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan MC, Yeung YC, Yu ELM, et al. Blood eosinophil and risk of exacerbation in chronic obstructive pulmonary disease patients: a retrospective cohort analysis. Int J Chron Obstruct Pulmon Dis 2020; 15: 2869–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med 2018; 198: 329–339. [DOI] [PubMed] [Google Scholar]
- 27. Cheng S-L, Lin C-H. Effectiveness using higher inhaled corticosteroid dosage in patients with COPD by different blood eosinophilic counts. Int J Chron Obstruct Pulmon Dis 2016; 11: 2341–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Couillard S, Larivée P, Courteau J, et al. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest 2017; 151: 366–373. [DOI] [PubMed] [Google Scholar]
- 29. DiSantostefano RL, Hinds D, Le HV, et al. Relationship between blood eosinophils and clinical characteristics in a cross-sectional study of a US population-based COPD cohort. Respir Med 2016; 112: 88–96. [DOI] [PubMed] [Google Scholar]
- 30. Duman D, Aksoy E, Agca MC, et al. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int J Chron Obstruct Pulmon Dis 2015; 10: 2469–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med 2018; 6: 747–758. [DOI] [PubMed] [Google Scholar]
- 32. Gonzalez-Barcala FJ, San-Jose ME, Nieto-Fontarigo JJ, et al. Blood eosinophils could be useful as a biomarker in chronic obstructive pulmonary disease exacerbations. Int J Clin Pract 2019; 73: e13423. [DOI] [PubMed] [Google Scholar]
- 33. Hasegawa K, Camargo CA., Jr. Prevalence of blood eosinophilia in hospitalized patients with acute exacerbation of COPD. Respirology 2016; 21: 761–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hegewald MJ, Horne BD, Trudo F, et al. Blood eosinophil count and hospital readmission in patients with acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2020; 15: 2629–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Landis S, Suruki R, Maskell J, et al. Demographic and clinical characteristics of COPD patients at different blood eosinophil levels in the UK clinical practice research datalink. COPD 2018; 15: 177–184. [DOI] [PubMed] [Google Scholar]
- 36. Lv M-Y, Qiang L-X, Li Z-H, et al. The lower the eosinophils, the stronger the inflammatory response? The relationship of different levels of eosinophils with the degree of inflammation in acute exacerbation chronic obstructive pulmonary disease (AECOPD). J Thorac Dis 2021; 13: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mendy A, Forno E, Niyonsenga T, et al. Blood biomarkers as predictors of long-term mortality in COPD. Clin Respir J 2018; 12: 1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nishimura K, Kusunose M, Sanda R, et al. Is blood eosinophil count a biomarker for chronic obstructive pulmonary disease in a real-world clinical setting? Predictive property and longitudinal stability in Japanese patients. Diagnostics 2021; 11: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oh Y-M, Lee KS, Hong Y, et al. Blood eosinophil count as a prognostic biomarker in COPD. Int J Chron Obstruct Pulmon Dis 2018; 13: 3589–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oshagbemi OA, Franssen FME, Braeken DCW, et al. Blood eosinophilia, use of inhaled corticosteroids, and risk of COPD exacerbations and mortality. Pharmacoepidemiol Drug Saf 2018; 27: 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Papi A, Dokic D, Tzimas W, et al. Fluticasone propionate/formoterol for COPD management: a randomized controlled trial. Int J Chron Obstruct Pulmon Dis 2017; 12: 1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet 2018; 391: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 43. Peng J, Yu Q, Fan S, et al. High blood eosinophil and YKL-40 levels, as well as low CXCL9 levels, are associated with increased readmission in patients with acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2021; 16: 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Poder TG, Carrier N, Bélanger M, et al. Eosinophil counts in first COPD hospitalizations: a 1-year cost analysis in Quebec, Canada. Int J Chron Obstruct Pulmon Dis 2018; 13: 3065–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Prins HJ, Duijkers R, Lutter R, et al. Blood eosinophilia as a marker of early and late treatment failure in severe acute exacerbations of COPD. Respir Med 2017; 131: 118–124. [DOI] [PubMed] [Google Scholar]
- 46. Roche N, Chapman KR, Vogelmeier CF, et al. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME trial. Am J Respir Crit Care Med 2017; 195: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 47. Serafino-Agrusa L, Scichilone N, Spatafora M, et al. Blood eosinophils and treatment response in hospitalized exacerbations of chronic obstructive pulmonary disease: a case-control study. Pulm Pharmacol Ther 2016; 37: 89–94. [DOI] [PubMed] [Google Scholar]
- 48. Singh D, Wedzicha JA, Siddiqui S, et al. Blood eosinophils as a biomarker of future COPD exacerbation risk: pooled data from 11 clinical trials. Respir Res 2020; 21: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Song JH, Lee C-H, Kim JW, et al. Clinical implications of blood eosinophil count in patients with non-asthma-COPD overlap syndrome COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 2455–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vedel-Krogh S, Nordestgaard BG, Lange P, et al. Blood eosinophil count and risk of pneumonia hospitalisations in individuals with COPD. Eur Respir J 2018; 51: 1800120. [DOI] [PubMed] [Google Scholar]
- 51. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet 2017; 389: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 52. Yun JH, Lamb A, Chase R, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2018; 141: 2037–2047.e2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang Y, Liang L-R, Zhang S, et al. Blood eosinophilia and its stability in hospitalized COPD exacerbations are associated with lower risk of all-cause mortality. Int J Chron Obstruct Pulmon Dis 2020; 15: 1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Global Initiative for Asthma. GINA-Main-Report-2021-V2-WMS, https://ginasthma.org/reports/ (accessed 2 June 2021).
- 55. Ferguson GT, Anzueto A, Fei R, et al. Effect of fluticasone propionate/salmeterol (250/50 microg) or salmeterol (50 microg) on COPD exacerbations. Respir Med 2008; 102: 1099–1108. [DOI] [PubMed] [Google Scholar]
- 56. Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med 2013; 1: 210–223. [DOI] [PubMed] [Google Scholar]
- 57. Sharafkhaneh A, Southard JG, Goldman M, et al. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir Med 2012; 106: 257–268. [DOI] [PubMed] [Google Scholar]
- 58. Suissa S. Number needed to treat in COPD: exacerbations versus pneumonias. Thorax 2013; 68: 540–543. [DOI] [PubMed] [Google Scholar]
- 59. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 60. Hinds DR, DiSantostefano RL, Le HV, et al. Identification of responders to inhaled corticosteroids in a chronic obstructive pulmonary disease population using cluster analysis. BMJ Open 2016; 6: e010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Oshagbemi OA, Odiba JO, Daniel A, et al. Absolute blood eosinophil counts to guide inhaled corticosteroids therapy among patients with COPD: systematic review and meta-analysis. Current Drug Targets 2019; 20: 1670–1679. [DOI] [PubMed] [Google Scholar]
- 62. Harries TH, Rowland V, Corrigan CJ, et al. Blood eosinophil count, a marker of inhaled corticosteroid effectiveness in preventing COPD exacerbations in post-hoc RCT and observational studies: systematic review and meta-analysis. Respir Res 2020; 21: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mathioudakis AG, Bikov A, Foden P, et al. Change in blood eosinophils following treatment with inhaled corticosteroids may predict long-term clinical response in COP. Eur Respir J 2020; 55: 1902119. [DOI] [PubMed] [Google Scholar]
- 64. Koutsokera A, Stolz D, Loukides S, et al. Systemic biomarkers in exacerbations of COPD: the evolving clinical challenge. Chest 2012; 141: 396–405. [DOI] [PubMed] [Google Scholar]
- 65. Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med 2018; 6: 117–126. [DOI] [PubMed] [Google Scholar]
- 66. Oshagbemi OA, Burden AM, Braeken DCW, et al. Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am J Respir Crit Care Med 2017; 195: 1402–1404. [DOI] [PubMed] [Google Scholar]
- 67. Landis SH, Suruki R, Hilton E, et al. Stability of blood eosinophil count in patients with COPD in the UK clinical practice research datalink. COPD 2017; 14: 382–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-taj-10.1177_20406223211028768 for Blood eosinophil count-guided corticosteroid therapy and as a prognostic biomarker of exacerbations of chronic obstructive pulmonary disease: a systematic review and meta-analysis by Tao Liu, Zi-Jian Xiang, Xiao-Meng Hou, Jing-Jing Chai, Yan-Li Yang and Xiao-Tong Zhang in Therapeutic Advances in Chronic Disease