FIGURE 1.
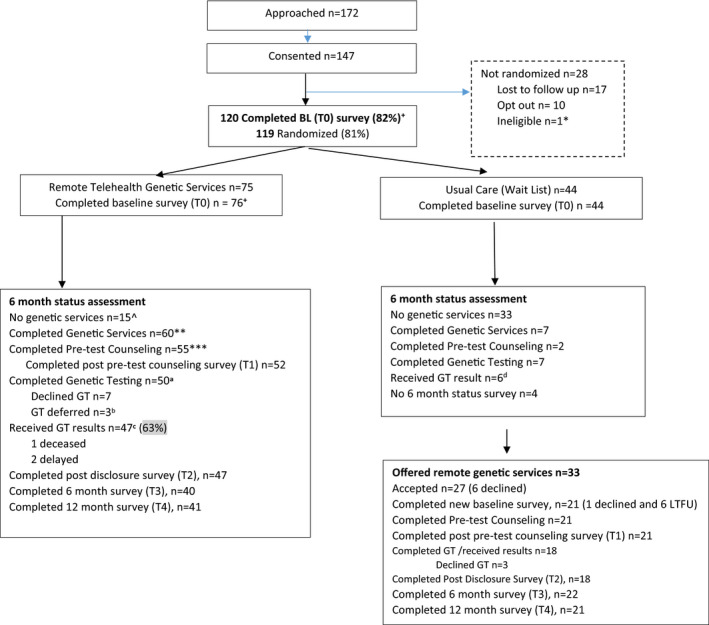
Study consort. *One enrolled participant was found to be ineligible and not randomized. +One participant completed T0 survey but we were unable to reach the participant to complete randomization. ++Randomization was initially 1:1:1 (remote phone: remote videoconference: usual care), but was changed to 1:1:2 to achieve adequate enrollment to meet our primary outcomes. ^One participant deceased. **Includes five participants who had external testing but not through our remote services (e.g. not per protocol). ***Does not include five participants who had external testing, because we can't confirm outside pre‐test counseling. a Includes five participants who had external testing and 45 remote participants who had V1 and blood draw within 6 months of randomization. bTesting deferred includes: (1) two participants who waited for relatives to test first and did not get genetic testing; one participant waited for mother's genetic testing but did finally have genetic testing through remote services. cOne participant died before results could be disclosed; two participants had disclosures at 7 and 8.8 months post randomization, respectively. dOne UC participant received results at 7.3 months
