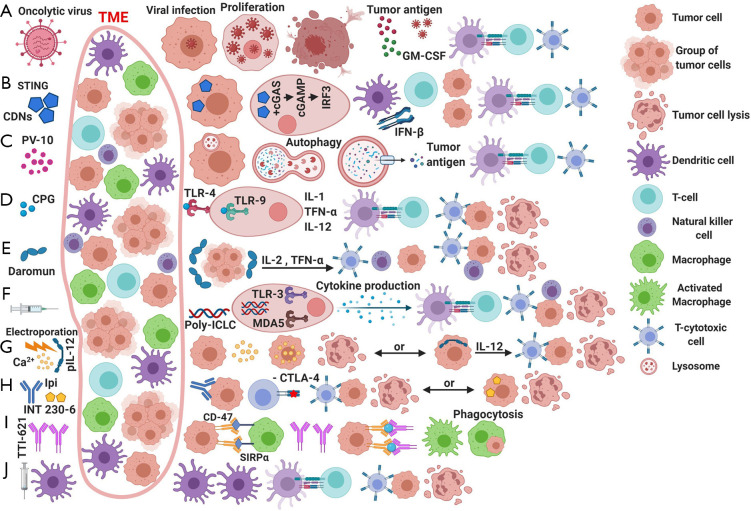
Figure 1.
Mechanism of action of different intralesional therapeutic regimens. (A) Oncolytic viruses pathway; T-VEC penetrate the cells, followed by virus proliferation and resultant cell lysis. Tumor antigens are made available through cytolysis, these antigens will be presented by DC and macrophages to cytotoxic CD8+ T lymphocytes, resulted in turn in activation of immune response against tumor cells. (B) STimulator of INterferon Genes (STING) pathway agonists; CDNs recognized by cGAS, resulting in the production of cGAMP, which in turn binds to STING molecule which will undergo a conformational change, subsequently resulting in a series of molecular interactions in the pathway of INF-β production. Increased INF-β expression will stimulate antigen-presenting cells (APCs) and increase the presence of infiltrating T-lymphocytes. (C) Rose Bengal-10 (PV-10). PV-10 preferentially absorbed by tumor cells to a larger extent in comparison to normal cells. Accumulating in the lysosomes, will then result in cell necrosis, release of tumor antigens, resulting in tumor specific immune response. (D) Toll-like receptors (TLRs) agonists: activating TLRs by CpG molecules will lead to stimulation of nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) pathway, leading to increase production of several chemokines and cytokines (e.g., IL-1, IL-12 and TNF-α) upregulating antigen presenting cells and increasing lymphocytes infiltration. (E) Intralesional interleukin-2 (IL-2) or Daromun administration: IL-2 potentiate T-helper cells differentiation and proliferation, cytolytic CD8+ T-cells and natural killer (NK) cells function, and also plays an important part in other cytokines production such as IL-9; (F) Intratumoral vaccines: Hiltonol (poly-ICLC) is able to produce an antitumor response by activating TLR-3 and MDA5, subsequently activating APCs, NK cells, T-cells, and upregulate the production of cytokines and chemokines. (G) Electroporation: by using short electrical pulses administered directly to the tumor, resulting in an increased permeability of tumor-cell walls to certain molecules (e.g., Ca2+ or pIL-12) that under normal circumstances are unable to penetrate the cell membrane. markedly increased intracellular calcium concentration results in tumor-cell necrosis due to sudden exhaustion of adenosine triphosphate (ATP) reserve. Or using the electroporation technology to introduce plasmid DNA that carries IL-12 gene, capable of transcribing RNA into tumor cells and ultimately increase IL-12 production resulting in an increased local immune response. (H) Intratumoral immunotherapy/chemotherapy; ipilimumab a human antibody directed against cytotoxic T lymphocyte associated antigen-4 receptor (CTLA-4), inhibiting CTLA-4 receptor will lead to an augmented local immune response by promoting T-cell activation and increase IL-2 production. Injected INT230-6 results in increased intracellular concentration of the combined chemotherapeutic agents (cisplatin and vinblastine) resulting in tumor cellular death, along with an increased concentration of immune cells (e.g., DC and T-cells) in the tumor microenvironment. (I) Other intratumoral agents: TTI-621 (SIRPαFc) protein: TTI-621 is recombinant human protein, that contains the N-terminal V domain of human SIRPα attached to an Fc region of human immunoglobulin G1 (IgG1). Administration of this agent will inhibit the CD47-SIRPα signal, resulting in an unblocked phagocytic function of the host macrophages against tumor cells. (J) Miscellaneous: dendritic cell therapy after cryotherapy in combination with pembrolizumab: injecting autologous vaccine using patient’s mature dendritic cells plus tumor proteins after undergoing lesion cryotherapy. Preceded by IV pembrolizumab. TME, tumor microenvironment; T-VEC, talimogene laherparepvec; GM-CSF, granulocyte monocyte-colony stimulating factor; CDN, cyclic dinucleotide; cGAS, cyclic GMP-AMP synthetase; cGAMP, cyclic GMP-AMP; IRF3, interferon regulatory transcription 3; PV-10, Rose Bengal-10; TLR, toll-like receptor; CpG, oligonucleotide with cytidine-phospho-guanosine patterns; NF-κB, nuclear Factor kappa-light-chain-enhancer of activated B pathway; IL-1, interleukin-1; IL-12, interleukin-12; TNF-α, tumor necrosis factor-alpha; Daromun (L19IL2 + L19TNF), interleukin-2 attached to L19 antibody fragment + tumor necrosis factor attached to L19 antibody fragment; poly-ICLC, a manufactured double-stranded RNA of polyinosinic-polycytidylic acid-poly-l-lysine carboxymethylcellulose; MDA5, melanoma differentiation associated protein 5; pIL-12, plasmid interleukin-12; Ipi, ipilimumab; INT230-6, cisplatin and vinblastine with an amphiphilic penetration enhancer; SIRPα, signal-regulatory protein alpha; CD47, cluster of differentiation 47.