Fig. 4.
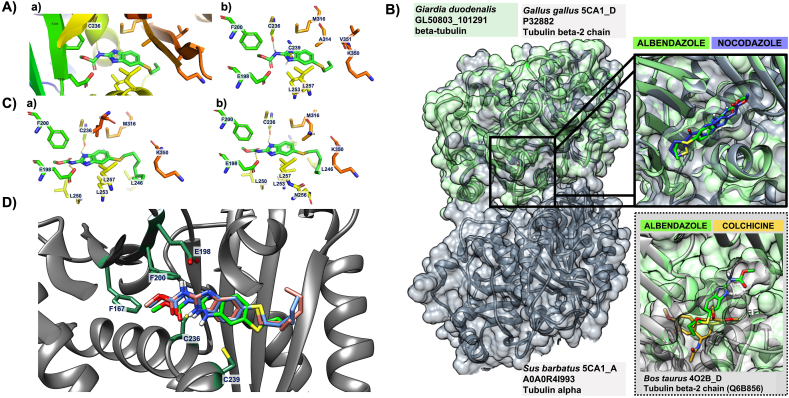
Molecular modelling of Alb with Giardia β-tubulin A) Illustrations of the binding mode of albendazole with Giardia β-tubulin. Surface view to represent binding pocket for Alb (left) and with the ribbon structure demonstrating polar contact (middle), with C236 and its hydrogen bond interaction indicated with an arror. The predicted binding modes of Alb inside the proposed binding site with the amino acid residues involved in hydrogen bond formation and non-bonded interaction are shown on the right. B) Overlapped binding sites of the experimentally derived crystal structure of T2R-TTL-nocodazole complex (PDB ID: 5CA1_C/D) shown in grey and our model of G. duodenalis β-tubulin shown in green. A close-up inserted image in the top right compared the orientation of nocodazole (blue) from the crystal structure (5CA1_D) and the docked Alb (green) structure from our molecular modelling of Giardia, and demonstrates that nocodozole and Alb share the same binding position and direction. In contrast, the inserted structure on the bottom left of the experimentally derived crystal structure of the tubulin-colchicine complex (PDB ID: 4O2B_C/D) in grey shows that the colchicine (yellow) molecule partially overlaps with docked Alb (green) in our structure from Giardia. C) Ribbon structures demonstrating polar contact between Giardia β-tubulin and heptylthioether (BI-63-8; left) and octyl-thiol (BI-63-14; right) analogues associated with decreased potency in the AlbR line. Orientation of the molecular is the same as in Alb, but feature an additional hydrogen bond with E198 for both molecules as indicated by the arrow.