Abstract
Purpose
The National Institutes of Health announced the Healthy Brain and Child Development (HBCD) study to further understanding of infant brain development. This study examined perceptions and knowledge about research among the demographic groups to be studied in HBCD.
Method
1164 participants (n = 548 pregnant people and 616 mothers of infants < 12 months) completed anonymous, on-line surveys. Domains included research literacy, MRI knowledge, and attitudes about research incentives and biospecimen collection. Logistic regression was used to examine factors related to outcome variables.
Results
Knowledge of MRI safety was low and research literacy was high across participants. Likelihood of participation given various incentives differed between participants. Those with lower education were less likely to rate any items as increasing likelihood of participation. Substance use during pregnancy improved the model fit only for items about alternate visit structures (home and telephone visits) and confidentiality.
Conclusion
Overall results support the feasibility of infant imaging studies, such as HBCD with respondents having high research literacy and interest in learning about their baby’s development. Educating potential participants about MRI safety and providing flexible incentives for participation will improve the success of infant MRI studies.
Keywords: Pregnant, Magnetic resonance imaging, Parent, Infant, Knowledge, Attitudes
1. Introduction
The prenatal environment is critical for healthy fetal brain development. Reported effects of substance use during pregnancy involve both adverse physical (i.e. low birth weight, decreased head circumference) and neurological (i.e. low arousal, poor self-regulation) outcomes (Nygaard et al., 2015; Shankaran et al., 2007; Thompson et al., 2009). Environmental factors that correspond with substance use during pregnancy, such as poverty, malnutrition, systemic racism, trauma, and stress, serve to both compound these effects and complicate our understanding of substances on the developing brain (Ellingson et al., 2012; Conradt et al., 2019).
Results from the 2019 National Survey on Drug Use and Health revealed that 18.4 % of responding US pregnant women indicated active use of illicit drugs, marijuana, tobacco products, or alcohol (Substance Abuse Mental Health Services Administration, 2020). Opioid use and dependence specifically have risen to epidemic levels since the 1990s, and consequently the number of infants born with neonatal abstinence syndrome (NAS) has also increased, escalating more than five-fold in the last ten years (Reddy et al., 2017). NAS is associated with impaired function across multiple body systems including the central and autonomic nervous systems, as well as the gastrointestinal and respiratory systems (Kakko et al., 2008).
Extant literature suggests that prenatal drug exposure produces short and long-term behavioral and neurodevelopmental consequences. The cognitive deficits associated with maternal use of alcohol and nicotine during pregnancy are widely known, including intellectual disabilities, attention deficits, motor hyperactivity, and learning deficits (Thompson et al., 2009). Similar risks have been identified when drugs, such as cocaine and amphetamines, are used during pregnancy (Shankaran et al., 2007; Thompson et al., 2009). Literature on the cognitive consequences of prenatal opioid exposure is mixed (see Conradt et al., 2019 for review), however there is evidence of reduced brain volume and altered brain structure among infants prenatally exposed to opioids (Monnelly et al., 2018; Sirnes et al., 2017).
However, existing research largely draws upon information related to birth outcomes (i.e. birth weight, head circumference, APGAR scores), with limited focus on longitudinal behavioral and neurodevelopmental outcomes. Additionally, the many confounding pre-and postnatal exposures that often occur alongside maternal substance use such as poverty, poly-substance use, and foster care placement, make it difficult to untangle causal factors. Further research is needed to fully understand the impact of substance exposure on the developing infant brain and to adequately identify and analyze the complex interplay between factors.
To further our knowledge of normative infant brain development and to increase understanding of alterations to trajectories associated with prenatal and early life events, the US National Institutes of Health conceived the Healthy Brain & Child Development (HBCD) Study (Volkow et al., 2020), which will enroll participants during pregnancy into a multi-modal, ten-year longitudinal study. The study will rigorously ascertain measures of brain, cognitive, social and emotional development, physical and biological development and will deeply phenotype the environment, beginning in utero. One focus of the HBCD study is to enroll pregnant people from environments associated with increased risk of negative developmental outcomes (in both substance using and non-substance using women), in addition to those from lower-risk environments (Volkow et al., 2020).
Many issues have been identified as foundational for the success of the HBCD study, and these issues are relevant more broadly for longitudinal studies of infants, and studies involving infant brain MRI. Designing the study to be attractive to pregnant people will be central to recruitment efforts. Once enrolled, issues such as the study visit structure, incentives provided by the study, and acceptability of research procedures will contribute to retention of families in the cohort. It is well known that study attrition is often biased, with families who have fewer resources also experiencing more negative life events that make continued study participation more challenging (Heinrichs et al., 2005; Kim et al., 2014). For example, lower income families are at heightened risk for housing instability (Phinney, 2013), which may challenge continued study enrollment. When study follow-up is biased, it threatens the internal validity of longitudinal data, and thus study retention must be considered as part of the overall study design. To understand barriers to participation, Beasley et al. (2020) conducted a qualitative study of pregnant women who were at high risk for substance use during pregnancy and found that logistical concerns (i.e. childcare, transportation) were a barrier to participation, and that having full information about the purpose and potential risks of the study was critical to interest in participation.
A central aspect of HBCD will be longitudinal MRI scanning. Use of Magnetic Resonance Imaging (MRI) has been demonstrated to be safe, including use in fetuses and neonates (Zvi et al., 2020). However, misperceptions about the safety of MRI, including that it exposes people to radiation, have been reported in Ghana and Nepal (Asante and Acheampong, 2020; Shrestha and Khadka, 2020). Examining perceptions of voluntary MRI studies in a US population of pregnant people is important to fully inform potential participants (an ethical obligation), and because it will affect study recruitment and retention. In addition, empirical data on participant preferences regarding visit structure and incentives and how these may differ between people will help to design studies such as HBCD to be of maximal benefit and to reduce participant burden.
While the existing literature provides general best-practices for these issues (see Beasley et al., 2020), data from geographically diverse people such as those who will participate in HBCD is missing. Thus, we sought to measure current attitudes, beliefs, and knowledge among the demographic groups relevant to HBCD (pregnant people and mothers of infants). The Investigation of Opioid Exposure and Neurodevelopment (iOPEN) consortium (Oregon Health & Science University, University of Vermont, New York University Langone Health, Icahn School of Medicine at Mount Sinai, and University of Pittsburgh Medical Center) sought to gain insights regarding the recruitment and retention of pregnant people who do and do not report using substances during pregnancy. We conducted an on-line survey of pregnant people and new mothers (with infants <12 months of age) during the Spring of 2020 across these five geographically diverse sites. The survey included items assessing MRI knowledge and willingness to participate in an MRI study, research literacy, preferences for research incentive/visit structure, and willingness and comfort with maternal and infant biospecimen collection. These domains were selected as they are central to planning HBCD, however they have broad relevance to studies of pregnant people and those with infants <12 months of age.
2. Material and methods
2.1. Participants
Pregnant people and their partners, and parents of an infant less than 12 months old were surveyed. Inclusion/exclusion criteria were minimal and included pregnancy/parenting status and age 18 or older.
2.2. Recruitment, survey distribution and completion
Participants were recruited from the geographic areas surrounding each of the five sites, and strategies included emails from treatment providers, social media, and snowball recruiting (see Rommel et al., this issue). Interested participants contacted the research team and were emailed a survey link. The survey link was not posted on social media or public websites, making contact with the study team required to receive the survey. Women with a history of substance use were oversampled via targeted recruitment material and recruitment from clinics specializing in providing prenatal care in the context of addiction.
Participants provided informed consent before data were collected, and the study was approved by the IRB at NYU, the single IRB for the iOPEN consortium. Survey responses were anonymous (i.e. not linked to personal identifiers). After completing the survey, identifying information needed to receive a $20 electronic gift card as compensation was collected using a separate on-line form.
2.2.1. The survey
The survey contained the following domains: demographics, mental health and substance use history, MRI knowledge and willingness, research literacy, incentive and visit structure preferences, and comfort with biospecimen collection.
Demographic questions included age, race, ethnicity, and educational attainment. Pregnant people reported their estimated due date and postpartum participants reported infant date of birth.
Mental Health, Substance Use, and Chronic Pain diagnoses (current and past) were self-reported, as was current and past treatment for each. To assess substance use during pregnancy, participants indicated how often (never, every day, few days a week, few days a month, less than once a month) they used each of 12 categories of substances during their most recent pregnancy. Substances included nicotine products, alcohol, marijuana, illicit opioids, medication for opioid use disorder, prescribed opioid pain medications, unprescribed opioid pain medications, cocaine, hallucinogens, amphetamines, prescribed benzodiazepines, and unprescribed benzodiazepines. Partners/fathers reported on the pregnant persons use during pregnancy.
MRI Knowledge was first assessed by asking participants whether they had ever heard of MRI. Response options were Yes, No, or Unsure. Participants then completed four true/false statements, including “MRI is safe for infants, children, and during pregnancy” and “MRI does not expose people to radiation.” Later in the survey, participants were informed that MRI was safe for babies and asked how often they would be willing to have a research visit that involved their baby having an MRI scan. Response options were every month, every 2–3 months, every 4–6 months, once in the first year, I would not be comfortable with this research visit, and I don’t know.
Research Literacy was queried by asking participants to indicate agreement (using a five-point scale from strongly agree to strongly disagree) with five statements addressing core constructs (informed consent, right to withdraw and the voluntary nature of research) as well as the purpose of medical research (to improve healthcare, importance of research). Responses were summarized into agree, neutral, and disagree for analyses.
Incentives and Research Practices were examined by asking participants to rate whether a given incentive would affect their likelihood of participating in a research study (using a five-point scale from much more likely to participate to much less likely to participate). Responses were summarized into more likely, neutral, and less likely for analyses. Incentives assessed included those intended to reduce the logistical burden of participation (free childcare during visits, coordinating with clinic visits, and offering home and telephone visits) and those providing education and social connectedness (learning about your baby’s development, free parenting workshops, free baby playgroups). In addition, confidentiality, not sharing information with child protective services, and participation of the child’s other biological parent were queried. Financial incentives were not queried as they are established effective reinforcers and are ubiquitous in research with this population to compensate participants for their time and effort participating in research.
Biospecimen Willingness was assessed separately for maternal and infant samples. Participants were given a list and asked to indicate any samples they were uncomfortable giving to researchers, or to indicate that they were comfortable with all of the samples in a group. Samples included their own and their infant’s blood, urine, saliva, feces, hair, and nails. Pregnant people were also asked about a vaginal swab, breast milk, placenta, and cord blood.
2.2.1.1. Data analysis
A descriptive approach was used to summarize participant responses. To examine differences between those who did and did not report substance use during pregnancy, groups were created for analysis. Demographic characteristics differed between these groups, therefore logistic regressions were used to assess group differences accounting for demographics. First, demographic variables (age, race, ethnicity, education level) were modeled to test for relationships with the outcome of interest, and then substance use group (report or no report of substance use during pregnancy) was added to see if model fit was improved. Demographic variable categories were collapsed (see Table 1) and the category with the largest number of participants was used as the reference category. Participants who declined to answer were omitted to address small cell numbers in the regressions. An a priori alpha of p < .05 was used for group comparisons.
Table 1.
Demographics of survey participants and comparison of those who did and did not report substance use during pregnancy.
| Demographics Frequencies for all response options are shown; grouping used for logistic regressions are noted, reference categories (Cat) noted with R and contain the largest number of respondents. | Participants |
||||
|---|---|---|---|---|---|
| Total Sample (n = 1164) | Participants who did NOT report substance use (n = 798) | Participants who DID report substance use (n = 284) | p-values from Chi Square group comparisons | ||
| Participant Type | Pregnant | 548 (47.1) | 424 (53.1) | 118 (41.5) | .001 |
| Mother | 616 (52.9) | 374 (46.9) | 166 (58.5) | ||
| Age | .001 | ||||
| Cat1:18–29 | 18−24 | 60 (5.2) | 37 (4.6) | 22 (7.7) | |
| Cat2:30–39R | 25−29 | 375 (32.2) | 254 (31.8) | 106 (37.3) | |
| Cat3:40+ | 30−34 | 492 (42.3) | 363 (45.5) | 89 (31.3) | |
| 35−39 | 184 (15.8) | 116 (14.5) | 53 (18.7) | ||
| 40−44 | 44 (3.8) | 25 (3.1) | 11 (3.8) | ||
| 45+ | 9 (0.8) | 3 (0.4) | 3 (1.1) | ||
| Race | <.001 | ||||
| Cat1:Black/African American | American Indian/ Alaskan Native | 9 (0.8) | 5 (0.6) | 4 (1.4) | |
| Cat2:White/Caucasian R | Asian | 23 (2.0) | 18 (2.3) | 2 (0.7) | |
| Cat3:More than 1 race | Black/African American | 126 (10.8) | 86 (10.8) | 33 (11.6) | |
| Cat4:Other | Native Hawaiian/ Pacific Islander | 4 (0.3) | 4 (0.5) | 0 | |
| White/Caucasian | 947 (81.4) | 658 (82.5) | 218 (76.7) | ||
| More than 1 race | 42 (3.6) | 17 (2.1) | 25 (8.8) | ||
| Other/ Decline | 13 (1.1) | 10 (1.3) | 2 (0.7) | ||
| Ethnicity | Not Hispanic/ Latinx | 1090 (93.6) | 757 (94.7) | 257 (90.5) | .031 |
| Cat1: Not Hispanic/Latinx R | Hispanic/ Latinx | 52 (4.5) | 29 (3.6) | 18 (6.3) | |
| Cat2: Hispanic/LatinX | Decline | 22 (1.9) | 12 (1.5) | 9 (3.2) | |
| Highest Education | Less than 12th grade | 24 (2.1) | 5 (0.6) | 19 (6.7) | <.001 |
| Cat1: Highschool or less | High school diploma | 74 (6.4) | 39 (4.9) | 31 (10.9) | |
| Cat2: Some college/2-year degree | GED | 29 (2.5) | 6 (0.8) | 23 (8.1) | |
| Cat3: Bachelor’s degree R | Some college | 135 (11.6) | 74 (9.3) | 57 (20.1) | |
| Cat4: Masters/Professional degree | 2-year degree | 225 (19.3) | 173 (21.7) | 48 (16.9) | |
| Bachelor’s degree | 417 (35.8) | 313 (39.2) | 67 (23.6) | ||
| Master’s degree | 186 (16.0) | 136 (17.0) | 29 (10.2) | ||
| Doctoral or professional degree | 71 (6.1) | 50 (6.3) | 9 (3.2) | ||
| Decline | 3 (0.3) | 2 (0.3) | 1 (0.4) | ||
Note. Data presented are n(%). Of the 424 pregnant people who did not report drug use, 300 (70.6 %) were pregnant for the first time. Of the 118 who did report drug use, 81 (68.6 %) were pregnant for the first time. GED = general educational development.
3. Results
3.1. Participants
A total of 1482 records were created by participants who completed the consent form. Nine-teen records were from participants who never began the survey and were excluded. Participants who were not pregnant or the mother of an infant < 12 months (n = 298; 160 partners of pregnant people and 138 fathers) were examined as a separate cohort and are reported descriptively in the Supplement. Given the small sample size, and general similarity of responses between the partners/fathers and the pregnant people/mothers, the partner/father data were not included in group comparisons, nor in regressions used to identify demographic factors related to survey responses. Responses of 1164 (n = 548 pregnant people and 616 mothers of an infant < 12 months of age) were analyzed as described above, and are presented as the “Total Sample” in this manuscript.
To determine if there were differences related to substance use during pregnancy, respondents were grouped into those who reported using at least one substance more than once a month during their pregnancy (n = 284; 24.4 %) and those who reported no substance use during pregnancy (n = 798; 68.6 %). Participants who used substances infrequently (once a month or less; n = 82; 7.0 %) were not included in these comparisons. Of those who reported substance use, 172 (60.3 %) reported a single substance and 112 (39.7 %) reported poly-substance use. The most frequently reported substances used were alcohol (39 % of single substance users and 43 % of poly-substance users); nicotine (25 % of single substance users and 79 % of poly-substance users, and marijuana (9 % of single substance users and 35 % of poly-substance users). Demographics of the total sample and those who did and those who did not report substance use are presented in Table 1. The substance use groups differed on all demographic characteristics, with more mothers than pregnant people reporting substance use during pregnancy. The group who reported no substance use included overrepresentation of White, Non-Hispanic/Latinx, and those with a Master’s degree or higher education level.
3.2. MRI knowledge and willingness
In the total sample, 92.4 % (n = 1076) had heard of MRI. Half (50.3 %) of participants believed MRI was safe for babies, and 46 % correctly endorsed that MRI did not use radiation. Participant responses are presented in Table 2.
Table 2.
Participant responses to true/false statements assessing MRI knowledge.
| MRI Knowledge |
Participants |
|||
|---|---|---|---|---|
| Total Sample (n = 1164) | Participants who did NOT report substance use (n = 798) | Participants who DID report substance use (n = 284) | ||
| MRI is safe for babies. | True | 585 (50.3) | 419 (52.5) | 132 (46.5) |
| False | 127 (10.9) | 83 (10.4) | 37 (13.0) | |
| Unsure | 452 (38.8) | 296 (37.1) | 115 (40.5) | |
| MRI is safe for children. | True | 683 (58.7) | 475 (59.5) | 160 (56.3) |
| False | 96 (8.2) | 65 (8.2) | 27 (9.5) | |
| Unsure | 385 (33.1) | 258 (32.3) | 97 (34.2) | |
| MRI does NOT expose people to radiation. | True | 536 (46.0) | 379 (47.5) | 122 (43.0) |
| False | 280 (24.1) | 199 (24.9) | 63 (22.2) | |
| Unsure | 348 (29.9) | 220 (27.6) | 99 (34.9) | |
| MRI is safe during pregnancy. | True | 452 (38.8) | 313 (39.2) | 111 (39.1) |
| False | 255 (21.9) | 179 (22.4) | 59 (20.8) | |
| Unsure | 457 (39.3) | 306 (38.4) | 114 (40.1) | |
Note. Data presented are n (%). All statements are true.
Logistic regression showed demographic factors (education and race) were related to responses, but substance use during pregnancy was not (see Table 3). Participants with lower education were more likely to respond “No” or “Unsure” to “Have you ever heard of MRI?” than those with a Bachelor’s degree. In addition, these participants were more likely to respond “False” or “Unsure” to all of the MRI safety questions. When presented with the statement “MRI is safe for babies,” those with a professional degree were more likely to reply “False” or “Unsure” than those with a Bachelor’s degree. Black (compared to White) participants were more likely to correctly respond “True” to MRI being safe for babies and pregnant people.
Table 3.
Logistic regressions for MRI knowledge questions.
| Model Fit | VARIABLE | B (SE) | OR (95 % CI) | p VALUE |
|---|---|---|---|---|
| Have you ever heard of MRI? (likelihood of No/Unsure response vs Yes) | ||||
| AIC = 542 | High school or less | 1.11 (0.40) | 3.03 (1.4–6.6) | 0.006 |
| Adj R2 = 0.077 | Some college/2-year degree | 0.87 (0.33) | 2.39 (1.2–4.6) | 0.009 |
| MRI does NOT expose people to radiation (likelihood of False/Unsure response vs True) | ||||
| AIC = 1436 | High school or less | 1.21 (0.24) | 3.3 (2.1–5.4) | <.001 |
| Adj R2 = 0.058 | Some college/2-year degree | 0.56 (0.15) | 1.8 (1.3–2.4) | <.001 |
| MRI is safe for babies (likelihood of False/Unsure response vs True) | ||||
| AIC = 1434 | Black | −0.57 (0.12) | 0.57 (0.4 – 0.9) | 0.008 |
| Adj R2 = 0.064 | High school or less | 1.27 (0.24) | 3.55 (2.2–5.7) | <.001 |
| Master’s/Professional | 0.35 (0.18) | 1.42 (1.0–2.0) | 0.044 | |
| MRI is safe for children (likelihood of False/Unsure response vs True) | ||||
| AIC = 1428 | High school or less | 0.85 (0.22) | 2.3 (1.5–3.6) | <.001 |
| Adj R2 = 0.028 | Some college/2-year degree | 0.41 (0.16) | 1.5 (1.1–2.1) | 0.009 |
| MRI is safe for pregnant women (likelihood of False/Unsure response vs True) | ||||
| AIC = 1392 | Black | −0.50 (0.21) | 0.6 (0.4 – 0.9) | 0.019 |
| Adj R2 = 0.058 | High school or less | 1.33 (0.26) | 3.8 (2.3–6.3) | <.001 |
| Some college/2-year degree | 0.51 (0.16) | 1.7 (1.2–2.3) | 0.001 | |
Note. Modeled variables included Race, Ethnicity, Education, and Age. Reference groups were the most frequent responses (Race = White; Education = Bachelor’s; Ethnicity = not Hispanic/Latinx; Age = 30–39). Models were run with and without substance use status, and only significant factors of the best fitting model are included in the table. AIC = Akaike Information Criterion; Adj = adjusted; B(SE) = Beta value (Standard Error); OR = odds ratio; CI = confidence interval.
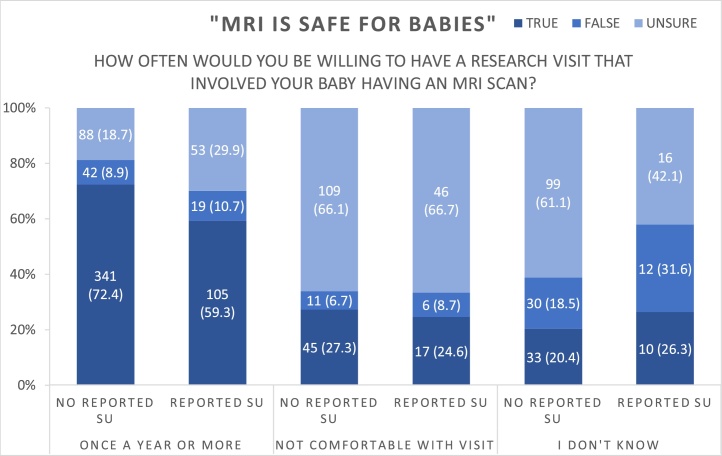
Comfort with research MRI scans for their infant was related to participant knowledge of MRI safety, with 80.3 % of women who agreed that MRI was safe for babies indicating they would participate in one or more infant MRI scans in the first year of life, compared to 48.8 % of those who felt MRI was unsafe and 34.7 % of those who were unsure about MRI safety [χ2 (4) = 248.0, p < .0001] (see Fig. 1). There were no significant differences by substance use group.
Fig. 1.
Willingness to participate in infant MRI shown across responses to “MRI is safe for babies” (true/false/unsure).
3.3. Research literacy
Research literacy was high among all participants with over 90 % endorsing agree/strongly agree to all domains queried (see Table 4).
Table 4.
Participant agreement/disagreement with statements about research.
| Research Literacy |
Participants |
|||
|---|---|---|---|---|
| Total Sample (n = 1164) | Participants who did NOT report substance use (n = 798) | Participants who DID report substance use (n = 284) | ||
| Research is important. | Agree | 1140 (97.9) | 786 (98.5) | 273 (96.1) |
| Neutral | 14 (1.2) | 3 (0.4) | 10 (3.5) | |
| Disagree | 10 (0.9) | 9 (1.1) | 1 (0.4) | |
| Research helps to make healthcare better for people in the future. | Agree | 1138 (97.8) | 783 (98.1) | 274 (96.5) |
| Neutral | 16 (1.4) | 6 (0.8) | 9 (3.2) | |
| Disagree | 10 (0.9) | 9 (1.1) | 1 (0.4) | |
| Being in a research study is voluntary, which means I don’t have to participate. | Agree | 1112 (95.5) | 765 (95.9) | 269 (94.7) |
| Neutral | 26 (2.2) | 14 (1.8) | 10 (3.5) | |
| Disagree | 26 (2.2) | 19 (2.4) | 5 (1.8) | |
| The researcher will tell me all the risks of being in the study before I start. | Agree | 1089 (93.6) | 755 (94.6) | 261 (91.9) |
| Neutral | 57 (4.9) | 31 (3.9) | 18 (6.3) | |
| Disagree | 18 (1.5) | 12 (1.5) | 5 (1.8) | |
| If I decide to stop being in a research study it is okay. | Agree | 1109 (95.3) | 764 (95.7) | 269 (94.7) |
| Neutral | 38 (3.3) | 21 (2.6) | 12 (4.2) | |
| Disagree | 17 (1.5) | 13 (1.6) | 3 (1.1) | |
Note. Data presented are n(%).
Logistic regressions (see Table 5) showed that having a high school education or less (compared with a Bachelor’s degree) was associated with a neutral response or disagreement to the statements that research improves healthcare in the future, and that all the risks would be disclosed before starting participation in a research study. Ethnicity was related to response on the voluntary nature of research, with Hispanic/Latinx respondents being more likely to be neutral or disagree with the statement “Being in a research study is voluntary, which means I don’t have to participate.” Substance use status did not improve the model fit for any items.
Table 5.
Logistic regressions for research literacy questions.
| Model Fit | VARIABLE | B (SE) | OR (95 % CI) | p VALUE |
|---|---|---|---|---|
| Research helps to make health care better for people in the future. | ||||
| Likelihood of neutral/disagree/strongly disagree vs agree/strongly agree | ||||
| AIC = 209 | High school or less | 1.86 (0.75) | 6.45 (1.5–27.9) | 0.013 |
| Adj R2 = 0.090 | ||||
| Being in a research study is voluntary, which means I don’t have to participate. | ||||
| Likelihood of neutral/disagree/strongly disagree vs agree/strongly agree | ||||
| AIC = 362 | Hispanic/Latinx | 1.04 (0.21) | 2.8 (1.0–7.8) | 0.042 |
| Adj R2 = 0.058 | ||||
| The researchers will tell me all the risks of being in the study before I start. | ||||
| Likelihood of neutral/disagree/strongly disagree vs agree/strongly agree | ||||
| AIC = 454 | High school or less | 1.36 (0.45) | 3.9 (1.6–9.5) | 0.003 |
| Adj R2 = 0.058 | ||||
Note. Modeled variables included Race, Ethnicity, Education, and Age. Reference groups were the most frequent responses (Race = White; Education = Bachelor’s; Ethnicity = not Hispanic/Latinx; Age = 30–39). Models were run with and without substance use status, and only significant factors of the best fitting model are included in the table. AIC = Akaike Information Criterion; Adj = adjusted; B(SE) = Beta value (Standard Error); OR = odds ratio; CI = confidence interval.
3.4. Incentive and visit structure preferences
The frequency of responses to incentive and visit structure preference questions is presented in Table 6. Incentives designed to reduce the burden of participation (make participating more convenient) were generally perceived to make study participation more likely. For example, 85 % of the sample responded that coordinating research visits with other doctor’s appointments would make participation more likely. Home visits were generally incentivizing (74.1 % endorsing more likely), however almost 7 % of respondent indicating that having the choice to do home visits would make their participation less likely. Learning about their baby’s development was appealing to most respondents, with 89 % indicating it would make participation more likely. Confidentiality and the assurance that information would not be shared with child protective services were rated as making participation more likely for approximately half of participants. While we do not know if these participants would have completed an infant brain imaging study, all participants were asked if they would like more information about participating in such a study. 75.8 % of survey respondents indicated they would like more information. Unfortunately, due to COVID-19 pandemic, research imaging studies were suspended at all sites during data collection.
Table 6.
Participant responses with regard to how different research incentives would influence their participation in a research study.
| Research Incentives |
Participants |
|||
|---|---|---|---|---|
| Total Sample (n = 1164) | Participants who did NOT report substance use (n = 798) | Participants who DID report substance use (n = 284) | ||
| If childcare was offered for my children while I am doing the study, I would be… | More likely | 888 (76.3) | 621 (77.8) | 209 (73.6) |
| No difference | 268 (23.0) | 173 (21.7) | 73 (25.7) | |
| Less likely | 8 (0.7) | 4 (0.5) | 2 (0.7) | |
| If my child's other biological parent was participating, I would be… | More likely | 726 (62.4) | 516 (64.7) | 176 (62.0) |
| No difference | 403 (34.6) | 260 (32.6) | 100 (35.2) | |
| Less likely | 35 (3.0) | 22 (2.8) | 8 (2.8) | |
| If research study visits were around the same time and at the same place as my other doctor's appointments, I would be… | More likely | 993 (85.3) | 690 (86.5) | 234 (82.4) |
| No difference | 159 (13.7) | 100 (12.5) | 46 (16.2) | |
| Less likely | 12 (1.0) | 8 (1.0) | 4 (1.4) | |
| If I had the choice to do some appointments by phone, I would be… | More likely | 1002 (86.1) | 709 (88.9) | 222 (78.2) |
| No difference | 143 (12.3) | 73 (9.2) | 59 (20.8) | |
| Less likely | 19 (1.6) | 16 (2.0) | 3 (1.0) | |
| If I had the choice to do some appointments in my home, I would be… | More likely | 862 (74.1) | 612 (76.7) | 191 (67.3) |
| No difference | 222 (19.1) | 134 (16.8) | 69 (24.3) | |
| Less likely | 80 (6.9) | 52 (6.5) | 24 (8.5) | |
| If I got to learn about my baby's development during the study, I would be… | More likely | 1041 (89.4) | 717 (89.9) | 246 (86.6) |
| No difference | 117 (10.1) | 76 (9.5) | 37 (13.0) | |
| Less likely | 6 (0.5) | 5 (0.6) | 1 (0.4) | |
| If I were invited to attend free parenting workshops, I would be… | More likely | 778 (66.8) | 540 (67.7) | 195 (68.7) |
| No difference | 350 (30.1) | 235 (29.5) | 76 (26.8) | |
| Less likely | 36 (3.1) | 23 (2.9) | 13 (4.6) | |
| If I were invited to attend a free baby playgroup, I would be… | More likely | 785 (67.4) | 558 (69.9) | 186 (65.5) |
| No difference | 345 (29.6) | 218 (27.3) | 87 (30.6) | |
| Less likely | 34 (2.9) | 22 (2.8) | 11 (3.9) | |
| If I knew that all of my information (including alcohol/drug use) would be kept confidential, I would be… | More likely | 723 (62.1) | 490 (61.4) | 192 (67.6) |
| No difference | 432 (37.1) | 300 (37.6) | 92 (32.3) | |
| Less likely | 9 (0.8) | 8 (1.0) | 0 | |
| If I knew that my substance use would not be reported to child protective services, I would be… | More likely | 572 (49.1) | 387 (48.5) | 165 (58.1) |
| No difference | 571 (49.1) | 398 (49.9) | 111 (39.1) | |
| Less likely | 21 (1.8) | 13 (1.6) | 8 (2.8) | |
Note. Data presented are n(%).
Logistic regressions predicting responses consistent with the item being incentivizing (i.e. it would make the participant more or much more likely to participate) versus being neutral or making participant less or much less likely to participate were run for each item (see Table 7). Black participants were 3.14 times more likely to indicate that providing childcare was incentivizing compared to White participants, while both participants with the lowest and highest levels of education (high school or less and Masters/Professional degree, respectively) were less likely than those with a four-year degree to endorse childcare as incentivizing participation.
Table 7.
Logistic regressions for offered research incentives.
| Model Fit | VARIABLE | B (SE) | OR (95 % CI) | p VALUE |
|---|---|---|---|---|
| If childcare was offered for my children while I am doing the study, I would be… | ||||
| Likelihood of more/much more likely vs no difference, less/much less likely to participate. | ||||
| AIC = 1105 | Black | 1.14 (0.33) | 3.14 (1.7–6.0) | <.001 |
| Adj R2 = 0.071 | High school or less | −1.14 (0.25) | 0.32 (0.2–0.5) | <.001 |
| Master’s/Professional | −0.45 | 0.64 (0.4–0.9) | 0.027 | |
| If research study visits were around the same time and at the same place as my other doctor’s appointments, I would be… | ||||
| Likelihood of more/much more likely vs no difference, less/much less likely to participate | ||||
| AIC = 835 | High school or less | −0.88 (0.18) | 0.57 (0.4–0.9) | 0.008 |
| Adj R2 = 0.069 | ||||
| If I had the choice to do some appointments by phone I would be… | ||||
| Likelihood of more/much more likely vs no difference, less/much less likely to participate | ||||
| AIC = 827 | Hispanic/Latinx | −0.76 (0.38) | 0.47 (0.2–1.0) | <.045 |
| Adj R2 = 0.047 | Highschool or less | −0.85 (0.28) | 0.42 (0.2–0.7) | 0.002 |
| *Above item with Group added to the model | ||||
| AIC = 820 | Hispanic/Latinx | −0.76 (0.38) | 0.47 (0.2–1.0) | <.045 |
| Adj R2 = 0.063 | Group = No Use | 0.62 (0.20) | 1.86 (1.3–2.8) | 0.002 |
| If I had the choice to do some appointments in my home I would be… | ||||
| Likelihood of more/much more likely vs no difference/less/much less likely to participate | ||||
| AIC = 1185 | High school or less | −0.88 (0.23) | 0.41 (0.3–0.7) | <.001 |
| Adj R2 = 0.036 | ||||
| Above item with Group added to the model | ||||
| AIC = 1184 | High school or less | −0.78 (0.24) | 0.46 (0.3–0.7) | 0.001 |
| Adj R2 = 0.038 | ||||
| If I got to learn about my baby’s development during the study I would be… | ||||
| Likelihood of more/much more likely vs no difference/less/much less likely to participate | ||||
| AIC = 681 | Hispanic/LatinX | −0.80 (0.40) | 0.45 (0.2–1.0) | 0.049 |
| Adj R2 = 0.069 | Highschool or less | −1.41 (0.32) | 0.25 (0.1–0.5) | <.001 |
| If I were invited to attend free parenting workshops, I would be… | ||||
| Likelihood of more/much more likely vs no difference/less/much less likely to participate | ||||
| AIC = 1246 | High school or less | −0.48 (0.24) | 0.62 (0.4–1.0) | 0.041 |
| Adj R2 = 0.120 | Master’s/Professional | −1.09 (0.18) | 0.34 (0.2–0.5) | <.001 |
| Age 40+ | −0.72 (0.34) | 0.49 (0.2–1.0) | 0.036 | |
| If I were invited to attend a free baby playgroup, I would be… | ||||
| Likelihood of more/much more likely vs no difference, less/much less likely to participate | ||||
| AIC = 1235 | Master’s/Professional | −1.03 (0.18) | 0.36 (0.2–0.5) | <.001 |
| Adj R2 = 0.115 | Black | 0.91 (0.28) | 2.48 (1.4–4.3) | 0.001 |
| If I knew that all of my information (including alcohol/drug use) would be kept confidential, I would be… | ||||
| Likelihood of more/much more likely vs no difference, less/much less likely to participate | ||||
| AIC = 1356 | Some college/2-year degree | 0.60 (0.17) | 1.82 (1.3–2.5) | <.001 |
| Adj R2 = 0.068 | Master’s/Professional | −0.43 (0.18) | 0.65 (0.5–0.9) | 0.015 |
| Above item with Group added to the model | ||||
| AIC = 1354 | Some college/2-year degree | 0.57 (0.17) | 1.77 (1.3–2.5) | <.001 |
| Adj R2 = 0.072 | Master’s/Professional | −0.43 (0.18) | 0.65 (0.5–0.9) | 0.016 |
| Black | 0.48 (0.24) | 1.61 (1.0–2.6) | 0.044 | |
| If I knew that my substance use would not be reported to child protective services, I would be… | ||||
| Likelihood of more/much more likely vs no difference, less/much less likely to participate | ||||
| AIC = 1349 | Some college/2-year degree | 0.69 (0.16) | 1.99 (1.5–2.7) | <.001 |
| Adj R2 = 0.162 | Master’s/Professional | −1.02 (0.19) | 0.36 (0.2–0.5) | <.001 |
| Age 40+ | −0.79 (0.38) | 0.45 (0.2–1.0) | 0.039 | |
| Black | 0.93 (0.24) | 2.53 (1.6–4.0) | <.001 | |
| If my child’s other biological parent was participating, I would be… | ||||
| Likelihood of more/much more likely vs no difference, less/much less likely to participate. | ||||
| AIC = 1259 | Black | 1.07 (0.27) | 2.91 (1.7–5.0) | <.001 |
| Adj R2 = 0.170 | More than one Race | −0.79 (0.38) | 0.45 (0.2–1.0) | 0.04 |
| High school or less | −0.98 (0.24) | 0.38 (0.2–0.6) | <.001 | |
| Master’s/Professional | −1.05 (0.18) | 0.35 (0.2–0.5) | <.001 | |
| Age <30 | 0.63 (0.16) | 1.88 (1.4–2.5) | <.001 | |
| Age 40+ | −0.85 (0.36) | 0.43 (0.2–1.0) | 0.02 | |
Note. Modeled variables included Race, Ethnicity, Education, and Age. Reference groups were the most frequent responses (Race = White; Education = Bachelor’s; Ethnicity = not Hispanic/Latinx; Age = 30–39). Models were run with and without substance use status, and only significant factors of the best fitting model are included in the table. AIC = Akaike Information Criterion; Adj = adjusted; B(SE) = Beta value (Standard Error); OR = odds ratio; CI = confidence interval.
Regarding telephone visits, adding substance use group to the model significantly improved the fit, and participants who reported no substance use during pregnancy were 1.86 times more likely to indicate that telephone visits would make their participation more likely. Hispanic/Latinx participants were less likely to indicate that telephone visits would incentivize study participation.
Both the lowest and highest education levels were less likely than those with a Bachelor’s degree to endorse free parenting workshops as incentivizing participation. Black respondents were 2.48 times more likely than White participants to rate free baby playgroups as making their study participation more likely.
The assurance of confidentiality and that substance use would not be reported to child protective services were associated with higher likelihood of participating among those with some college/two-year degree compared to those with a Bachelor’s degree and among Black (compared to White) participants.
3.5. Bio-sample comfort
Biospecimen comfort differed across the 15 sample types (see Table 8). Fewer than 15 % of respondants were uncomfortable providing their urine, saliva and nails; and their infants feces, saliva, and nails. However only 42 % of participants were comfortable providing all samples. The greatest discomfort was providing maternal feces (32.7 %) and infant blood (28.3 %). Demographic factors that were associated with biospecimen discomfort included age, race, and education, with those who were youngest (age <30 vs age 30–39), more than one race (compared to White), and with the highest education level (Master’s/Professional compared to Bachelor’s) being more likely to be uncomfortable providing one or more bio-samples. Participants with some college/two-year degrees and those who were Black were less likely to endorse discomfort providing bio-samples overall (see Table 9).
Table 8.
Participant discomfort with biospecimen collection, by sample type.
| Comfort with Biospecimen Samples (Frequencies represent those who were UNCOMFORTABLE providing each sample) |
Participants |
|||
|---|---|---|---|---|
| Total Sample (n = 1164) | Participants who did NOT report substance use (n = 798) | Participants who DID report substance use (n = 284) | ||
| ALL Samples | NO discomfort | 492 (42.3) | 340 (42.6) | 121 (42.6) |
| General adult samples | Blood | 202 (17.4) | 122 (15.3) | 63 (22.2) |
| Urine | 144 (12.4) | 78 (9.8) | 54 (19.0) | |
| Saliva | 128 (11.0) | 68 (8.5) | 48 (16.9) | |
| Feces | 386 (33.2) | 263 (33.0) | 91 (32.0) | |
| Hair | 210 (18.0) | 136 (17.0) | 59 (20.8) | |
| Nails | 170 (14.6) | 108 (13.5) | 47 (16.5) | |
| NO discomfort | 644 (55.3) | 449 (56.3) | 154 (54.2) | |
| Specific maternal samples | Vaginal swab | 259 (22.3) | 183 (22.9) | 63 (22.2) |
| Breast Milk | 160 (13.7) | 87 (10.9) | 61 (21.5) | |
| Placenta & cord blood | 253 (21.7) | 189 (23.7) | 45 (15.8) | |
| NO discomfort | 747 (64.2) | 517 (64.8) | 177 (62.3) | |
| Baby samples | Blood | 329 (28.3) | 239 (29.9) | 66 (23.2) |
| Urine | 181 (15.5) | 102 (12.8) | 66 (23.2) | |
| Saliva | 155 (13.3) | 90 (11.3) | 52 (18.3) | |
| Feces | 171 (14.7) | 97 (12.2) | 58 (20.4) | |
| Hair | 196 (16.8) | 133 (16.7) | 53 (18.7) | |
| Nails | 171 (14.7) | 114 (14.3) | 46 (16.2) | |
| NO discomfort | 702 (60.3) | 488 (61.2) | 165 (58.1) | |
Note. Data presented are n(%). “NO discomfort” captures the participants who are comfortable providing all above samples. Response type is multiple choice.
Table 9.
Logistic regressions for comfort with biospecimen samples.
| Model Fit | VARIABLE | B (SE) | OR (95 % CI) | p VALUE |
|---|---|---|---|---|
| Discomfort with one or more biospecimen samples. Likelihood of discomfort vs comfortable with all samples | ||||
| AIC = 2704 Adj R2 = 0.873 | Age <30 | 0.57 (0.13) | 1.74 (1.4–2.3) | <.001 |
| Black | −0.78 (0.20) | 0.46 (0.3–0.7) | <.001 | |
| More than one Race | 0.65 (0.32) | 1.92 (1.0–3.6) | 0.045 | |
| Other Race | 0.68 (0.31) | 1.98 (1.1–3.6) | 0.025 | |
| Some college/2-year degree | −0.69 (0.14) | 0.50 (0.4–0.7) | <.001 | |
| Master’s/Professional | 0.32 (0.16) | 1.38 (1.0–1.9) | 0.041 | |
Note. Modeled variables included Race, Ethnicity, Education, and Age. Reference groups were the most frequent responses (Race = White; Education = Bachelor’s; Ethnicity = not Hispanic/Latinx; Age = 30–39). Models were run with and without substance use status, and only significant factors of the best fitting model are included in the table. AIC = Akaike Information Criterion; Adj = adjusted; B(SE) = Beta value (Standard Error); OR = odds ratio; CI = confidence interval.
4. Discussion
The Healthy Brain and Child Development (HBCD) study is poised to be a landmark study characterizing normal infant brain development, mapping brain/behavior trajectories from infancy to childhood, and allowing examination of risk and protective factors. Given the importance of this study, it will be critical to enroll a diverse cohort, including those historically underrepresented in research, so that valid inferences can be made that apply to the population as a whole. The survey administered by the Investigation of Opioid Exposure and Neurodevelopment (iOPEN) consortium was designed to understand factors likely to affect research participation, including research literacy, comfort and knowledge with likely protocol elements, and examination of attitudes about incentives for participation. Key results support the ethics of engaging both women who do and do not report using substances during pregnancy into a study such as HBCD; highlight the importance of education around the safety of neonatal MRI to support recruitment efforts; provide reassurance with regard to the level of research literacy, and identify both broadly appealing incentives for participation (such as learning about their baby’s development) and those that vary with demographics and reported substance use during pregnancy. Partners of participants, and fathers of infants <12 months of age provided responses that are consistent with the pregnant people and mothers. These findings are relevant beyond the HBCD study and may inform the design of infant neuroimaging, and longitudinal studies of families of infants in the future.
While almost all participants were familiar with MRI, the finding that knowledge surrounding the safety of the procedure was low is notable. Only approximately half of participants correctly responded that MRIs were safe for babies. Perception of MRI safety was associated with participants’ stated willingness to participate in research MRI scans for their baby, highlighting the need to provide accurate information to families who are being recruited into a study with longitudinal MRI scans. While knowledge of MRI safety did not differ based on reported substance use during pregnancy, there was a relationship between education level and knowledge of MRI safety for babies. Interestingly, both those with the lowest and highest levels of education were more likely to be uncertain or to disagree with the statement that MRI is safe for babies. Thus, education on the safety of MRI should be provided to all potential HBCD participants, rather than assuming those with higher education are knowledgeable of and comfortable with the procedure.
The high levels of research literacy among participants stand in contrast to participant MRI knowledge. Over 90 % of participants agreed that research is important, improves healthcare, is voluntary, and that all risks are disclosed prior to participation, which can be stopped at any time. For those who may be concerned about the ethics of engaging perinatal women generally, and those who report substance use during pregnancy specifically, into studies such as HBCD, these data will be reassuring. However, participants with a high school education or less were 3.9 times as likely to not agree (to be neutral or to disagree) with the statement that they would be fully informed of the risks before participation in a study. In addition, Hispanic/Latinx participants were 2.8 time more likely than non-Hispanic/Latinx participants to not agree that research is voluntary. These findings indicate that historically underrepresented participants in research have less knowledge of and/or trust in the underlying ethical principles of research. Thus, care will need to be taken to ensure an accessible informed consent process is provided to all HBCD participants.
Overall, there was high research literacy in this sample may represent sample bias (i.e. only participants interested in research at some level would agree to complete a research survey). Participants were asked if they had previously participated in a research study and 27 % indicated that they had suggesting that this is not a primary driver of the high research literacy levels. Another consideration is that participants completed an informed consent process immediately prior to beginning this survey (although research literacy was not the first section of the survey). However, answers to many of the research literacy questions (i.e. voluntary nature of research, right to withdrawal, etc. had been presented factually to participants during the informed consent process. Additionally, it may be that research literacy is generally high in this population.
Once the HBCD cohort is recruited, efforts will shift to ensuring high levels of participant retention. Among the incentives that were queried, “learning about your baby’s development” was rated as incentivizing by a large majority of participants, indicating that providing parents with specific feedback about their infant will help retain subjects in HBCD. However, participants with the lowest education (high school or less) were less likely to rate all options as incentivizing. This is consistent with prior literature showing differences in participant retention by socioeconomic status (Heinrichs et al., 2005; Kim et al., 2014). It may be that both increased burdens and incentives that are less appealing contribute to higher study drop out among those with the fewest resources. Our findings suggest a lower incentive value of traditional incentives for those with low education levels. Another known factor affecting study retention among low-income families is the higher rate of life disrupting events (Heinrichs et al., 2005; Kim et al., 2014). Strategies such as developing flexible incentives in partnership with participants may help to avoid differential study drop out.
Of note, Black participants (compared to white participants) were more likely to rate several incentives as making participation more likely. These included free baby playgroups and childcare during study appointments; participation of the child’s other biological parent in the study; and assurances regarding confidentiality and not sharing sensitive information with child protective services. These findings are important and provide guidance for incentives to offer to maximize recruitment and retention of participants who identify as Black. Under-representation of minorities in clinical research has been well documented, (Killien et al., 2000; Yancey et al., 2006; Shavers et al., 2002; Shavers‐Hornaday et al., 1997) Mistrust of research and/or healthcare is one factor that may affect willingness to participate (Killien et al., 2000). This idea is consistent with Black participants rating confidentiality and non-disclosure to child protective services as more incentivizing than white participants. These assurances may be more salient among those who routinely encounter systemic oppression.
However, literature investigating willingness to participate in health research is complex. While many trials document lower participation of minorities, recent conceptualizations stress that structural factors such as whether a person is invited to participate, and whether circumstances such as the flexibility of an employer, the reliability of childcare, availability of transportation to the research site may influence participation more than willingness (Wendler et al., 2006). This notion is also consistent with our data as incentives such as childcare, free baby playgroups, and having the other biological parent participate were more likely to be selected as incentivizing by Black (vs white) participants. Taken together we found important differences for Black participants compared to white participants that suggest highlighting certain incentives in the design of HBCD.
Additionally, some protocol design choices, such as home visits – which are intended to reduce the burden of participating – were rated by a substantial portion (6.9 %) of participants as making participation less likely. This is interesting since it is known that alternative, flexible research visits can aid participation completion and retention, however it may be that participants who have had negative experiences with child protective services or other agencies are wary of opening their homes to researchers. In addition, research practices such as ensuring privacy and confidentiality when possible were rated as increasing the likelihood of study participation for about half of participants. Taken together, study visit structures and incentives should be as flexible as possible, with incentives tailored for each family. Combining tailored incentives with those that are broadly reinforcing (such as feedback on their infants’ development) will give researchers the best chance of recruiting and retaining a diverse sample in the HBCD study.
Limitations of the current study include the use of self-report to measure substance using during pregnancy. However, participant responses were anonymous, and no interaction other than survey completion and compensation were required. The finding that more women who were parents of young children reported retrospective substance use during pregnancy than did women who were currently pregnant may reflect bias in reporting, but this cannot be determined from the data collected. Additionally, the current study examined prenatal substance use by comparing those who did and did not report substance use during pregnancy, rather than interrogating specific substances or patterns of drug use. This is consistent with the proposed design of HBCD, which will examine naturalistic patterns of substance use (with oversampling those with high propensity for drug use). The survey that was used is not a validated instrument, which may limit the generalizability of these findings. Because the sample was drawn from areas surrounding the 5-sites, not all geographic regions of the country are represented. In addition, the sample had overrepresentation of people with a college degree, which may reflect the on-line nature of the survey (i.e. the survey was available to those who are on-line and comfortable with the text-dense nature on electronic consent and on-line surveys). In addition, because the survey was completed as research, the sample is skewed towards those with at least some interest and comfort with participating in research. How this affected the survey responses is unknown.
5. Conclusions
This study provides important evidence supporting the ethics of engaging perinatal women, including those who report substance use during pregnancy, in a study such as HBCD. While the majority of potential participants will have foundational research literacy, providing education on MRI safety will be critical to successful recruitment in HBCD. Research incentives will also be important in recognizing and balancing the burden of participation. However, research burden is not equally shared by all participants, and research incentives are not uniformly incentivizing. Therefore, providing incentives that are personally valuable for all participants will be necessary to minimize differential drop out of those with fewer resources. In addition, highlighting incentives that are of value to almost all participants, such as feedback on their baby’s development and coordinating with medical appointments, will broadly help study retention. By taking these overall themes into account, future brain development studies will better represent diverse groups of perinatal women.
Data statement
During the informed consent process, participants were informed their raw data would not be shared.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding: This work was supported by the National Institutes of Health [grant numbers 1R34DA050291-01, 1R34DA050290-01, 1R34DA050283-01, 1R34DA050287-01].
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100986.
Contributor Information
Kaelyn L. Kohlasch, Email: kaelyn.kohlasch@uvm.edu.
Leigh-Anne Cioffredi, Email: leigh-anne.cioffredi@uvmhealth.org.
Carly Lenninger, Email: carly.lenninger@nyulangone.edu.
Ellen Stewart, Email: stewartep@mwri.magee.edu.
Tessa Vatalaro, Email: tessa.vatalaro@nyulangone.org.
Hugh Garavan, Email: hgaravan@uvm.edu.
Alice Graham, Email: grahaal@ohsu.edu.
Sarah H. Heil, Email: sarah.heil@uvm.edu.
Elizabeth E. Krans, Email: kransee@upmc.edu.
Thalia Robakis, Email: thalia.robakis@mssm.edu.
Anna Rommel, Email: anna.rommel@mssm.edu.
Elinor L. Sullivan, Email: sullivel@ohsu.edu.
Moriah Thomason, Email: Moriah.Thomason@nyulangone.edu.
Alexandra Potter, Email: Alexandra.Potter@uvm.edu.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Asante S., Acheampong F. Patients’ knowledge, perception, and experience during magnetic resonance imaging in Ghana: a single centre study. Radiography (Lond) 2020 doi: 10.1016/j.radi.2020.11.020. [DOI] [PubMed] [Google Scholar]
- Beasley L.O., Ciciolla L., Jespersen J.E., Chiaf A.L., Schmidt M., Shreffler K.M., Breslin F.J., Nakhireva L.N., Sanjuan P.M., Stephen J.M., Coles C.D., Chambers C.D., Kable J.A., Leeman L., Singer L.T., Zellner J., Morris A.S., Croff J.M. Best practices for engaging pregnant and postpartum women at risk of substance use in longitudinal research studies: a qualitative examination of participant preferences. Advers. Resil. Sci. 2020;1(4):235–246. doi: 10.1007/s42844-020-00019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E., Flannery T., Aschner J.L., Annett R.D., Croen L.A., Duarte C.S. Prenatal opioid exposure: neurodevelopmental consequences and future research priorities. Pediatrics. 2019;144(3) doi: 10.1542/peds.2019-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson J.M., Rickert M.E., Lichtenstein P., Långström N., D’Onofrio B.M. Disentangling the relationships between maternal smoking during pregnancy and co-occurring risk factors. Psychol. Med. 2012;42(7):1547. doi: 10.1017/S0033291711002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs N., Bertram H., Kuschel A., Hahlweg K. Parent recruitment and retention in a universal prevention program for child behavior and emotional problems: barriers to research and program participation. Prev. Sci. 2005;6(4):275–286. doi: 10.1007/s11121-005-0006-1. [DOI] [PubMed] [Google Scholar]
- Kakko J., Heilig M., Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96(1–2):69–78. doi: 10.1016/j.drugalcdep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Killien M., Bigby J.A., Champion V., Fernandez-Repollet E., Jackson R.D., Kagawa-Singer M., Kidd K., Naughton M.J., Prout M. Involving minority and underrepresented women in clinical trials: the National Centers of Excellence in Women’s Health. J. Womens Health Gend. Med. 2000;9:1061–1070. doi: 10.1089/152460900445974. [DOI] [PubMed] [Google Scholar]
- Kim R., Hickman N., Gali K., Orozco N., Prochaska J.J. Maximizing retention with high-risk participants in a clinical trial. Am. J. Health Promot. 2014;28(4):268–274. doi: 10.4278/ajhp/120720-QUAN-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnelly V.J., Anblagan D., Quigley A., Cabez M.B., Cooper E.S., Mactier H. Prenatal methadone exposure is associated with altered neonatal brain development. Neuroimage Clin. 2018;18:9–14. doi: 10.1016/j.nicl.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard E., Moe V., Slinning K., Walhovd K.B. Longitudinal cognitive development of children born to mothers with opioid and polysubstance use. Pediatr. Res. 2015;78(3):330–335. doi: 10.1038/pr.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney R. Exploring residential mobility among low-income families. Soc. Serv. Rev. 2013;87(4):780–815. doi: 10.1086/673963. [DOI] [Google Scholar]
- Reddy U.M., Davis J.M., Ren Z., Greene M.F. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstet. Gynecol. 2017;130(1):10–28. doi: 10.1097/AOG.0000000000002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel A., et al. (This Issue) Approaches to the recruitment of pregnant women who use substances for participation in research.
- Shankaran S., Lester B.M., Das A., Bauer C.R., Bada H.S., Lagasse L., Higgins R. Impact of maternal substance use during pregnancy on childhood outcome. Semin. Fetal Neonatal Med. 2007;12(2):143–150. doi: 10.1016/j.siny.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavers V.L., Lynch C.F., Burmeister L.F. Racial differences in factors that influence the willingness to participate in medical research studies. Ann. Epidemiol. 2002;12:248–256. doi: 10.1016/s1047-2797(01)00265-4. [DOI] [PubMed] [Google Scholar]
- Shavers‐Hornaday V.L., Lynch C.F., Burmeister L.F., Torner J.C. Why are African Americans under-represented in medical research studies? Impediments to participation. Ethn. Health. 1997;2:31–45. doi: 10.1080/13557858.1997.9961813. [DOI] [PubMed] [Google Scholar]
- Shrestha S., Khadka B. Assessment of patients’ knowledge, perception and safety regarding MRI scan. J. Manmohan Meml. Inst. Health Sci. 2020;6(1):4–20. [Google Scholar]
- Sirnes E., Oltedal L., Bartsch H., Eide G.E., Elgen I.B., Aukland S.M. Brain morphology in school-aged children with prenatal opioid exposure: a structural MRI study. Early Hum. Dev. 2017;106-107:33–39. doi: 10.1016/j.earlhumdev.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Substance Abuse Mental Health Services Administration . 2020. National Survey on Drug Use and Health 2019.https://www.samhsa.gov/data/report/2019-nsduh-detailed-tables (September 11) [Google Scholar]
- Thompson B.L., Levitt P., Stanwood G.D. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat. Rev. Neurosci. 2009;10(4):303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Gordon J.A., Freund M.P. The healthy brain and child development study—shedding light on opioid exposure, COVID-19, and health disparities. JAMA Psychiatry. 2020 doi: 10.1001/jamapsychiatry.2020.3803. [DOI] [PubMed] [Google Scholar]
- Wendler D., Kington R., Madans J., Wye G.V., Christ-Schmidt H. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;2(3) doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey A.K., Ortega A.N., Kumanyika S.K. Effective recruitment and retention of minority research participants. Annu. Rev. Public Health. 2006;27:1–28. doi: 10.1146/annurev.publhealth.27.021405.102113. [DOI] [PubMed] [Google Scholar]
- Zvi E., Shemer A., Toussia-Cohen S., Zvi D., Bashan Y., Hirschfeld-Dicker L., Oselka N., Amitai M.M., Ezra O., Bar-Yosef O., Katorza E. Fetal exposure to MR imaging: long-term neurodevelopmental outcome. AJNR Am. J. Neuroradiol. 2020;41(11):1989–1992. doi: 10.3174/ajnr.A6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.