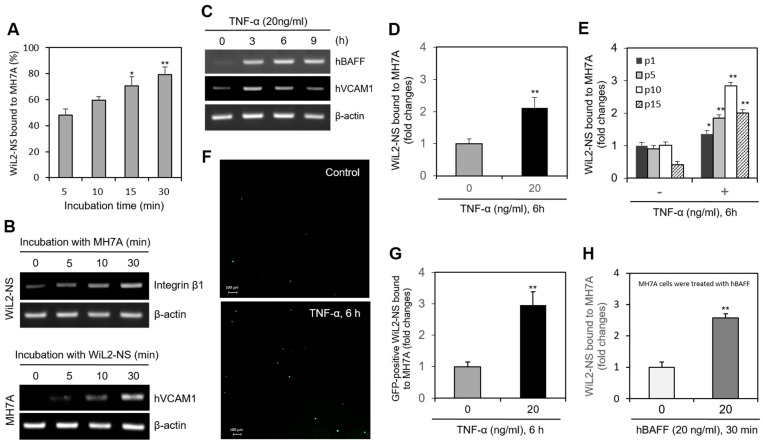
Figure 1.
TNF-α enhanced a binding of B cells to synovial cells. (A,B) MH7A cells were plated overnight. Then, WiL2-NS cells were added and co-incubated to adhere for 5, 10, 15 and 30 min (A). Each cell was collected separately at each time point. RNA was purified with Nucleozol®. The expression of human vascular cell adhesion molecule-1 (hVCAM1) and integrin β1 in MH7A and WiL2-NS cells was respectively detected by RT-PCR (B). (C) Primary synoviocytes were treated with 20 ng/mL TNF-α for various times. RNA was purified with Nucleozol®. The expression of hBAFF and hVCAM1 was detected by RT-PCR. (D,E) MH7A cells were plated overnight and treated with 20 ng/mL TNF-α for 6 h. Then, WiL2-NS cells were added and co-incubated to adhere for 30 min. Unbound WiL2-NS B cells were washed out and MTT assay was used to analyze bound B cells (D). WiL2-NS cell adhesion to MH7A cells of each passage were measured by MTT assay (E). (F,G) WiL2-NS cells were transfected with pSG5-GFP plasmid DNA by using Viafect®. GFP-positive WiL2-NS cells were visualized under fluorescence microscope (F). Transfected WiL2-NS cells were co-incubated with MH7A cells at the ratio of 1:1 for 30 min. Unbound WiL2-NS B cells were washed out. Then, GFP-positive WiL2-NS cells were counted to analyze bound B cells (G). (H) MH7A cells were treated with hBAFF protein for 30 min. Then, WiL2-NS cells were added and co-incubated to adhere for 30 min. Unbound WiL2-NS B cells were washed out and MTT assay was used to analyze bound B cells. Each experiment was performed at least four times. Data in a bar graph represent the means ± SD. * p < 0.05, ** p < 0.01; significantly different from group with 5 min-incubation (A), TNF-α- (D,E,G) or hBAFF- (H) untreated control group.