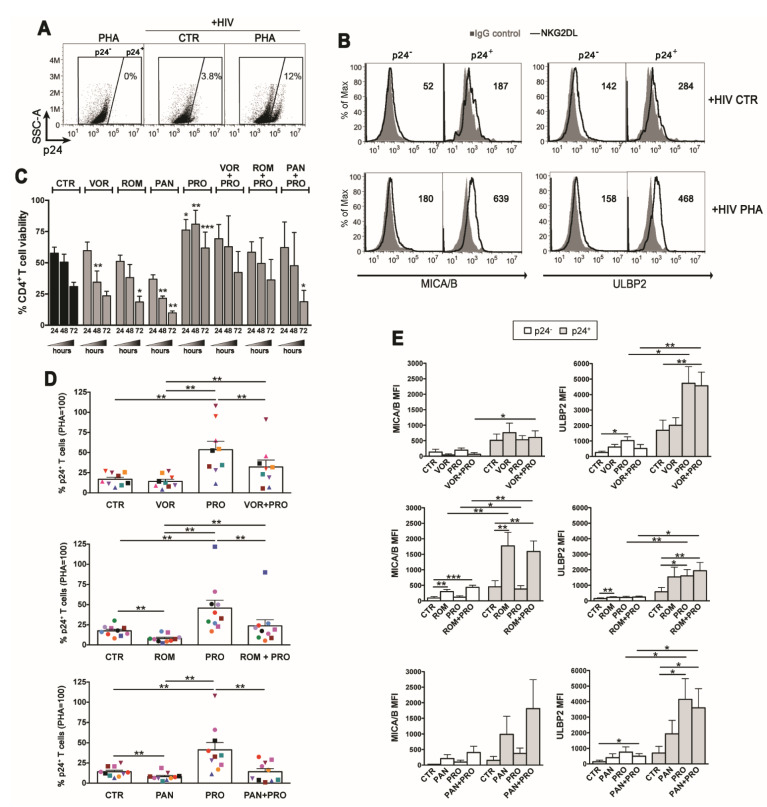
Figure 3.
Impact of the HDACi/PRO combinations in viability, viral reactivation, and NKG2DLs expression in latently HIV-infected CD4+ T cells. HIV latency was established in CD4+ T cells freshly isolated from healthy donors (see Materials and Methods), then cells were exposed to LRAs for 3 days prior 2-color flow cytometry analysis of cell-surface MICA/B and ULBP2 expression in p24- and p24+ cells. (A) Representative dot plots show the frequency of p24+ cells in control (CTR) and PHA-stimulated cultures gated by setting non-infected PHA-stimulated cells at 0%. (B) Histograms show MICA/B and ULBP2 fluorescence (solid line) in gated p24- and p24+ cell populations measured in representative control and PHA-stimulated cell samples. Signals obtained with control IgG (filled histograms) and the ligand-specific MFI value, subtracted of the MFI value of isotype control, are shown. (C–E) Cells of several donors were cultivated in absence of stimuli (CTR) or with VOR (334 nM), ROM (10 nM), PAN (20 nM), PRO (1 µM), or combinations of each HDACi with PRO. (C) T cell viability (mean ± SEM, n = 3) was examined at 24, 48, and 72 h by LIVE/DEAD staining. (D) The frequency of p24+ cells harboring reactivated virus was evaluated for all conditions in 9–10 independent experiments (each donor is represented with distinct symbol) and expressed relatively to PHA set at 100%. (E) The cell-surface MICA/B and ULBP2 expression was analyzed on p24- and p24+ cells as described in (B) in 5–8 independent experiments. Bars represent mean ± SEM. Statistics was performed using paired t-test (C and E panels) and Wilcoxon test (D panel) for parametric and non-parametric distributions, respectively. * p < 0.05, ** p < 0.01, *** p < 0.001.