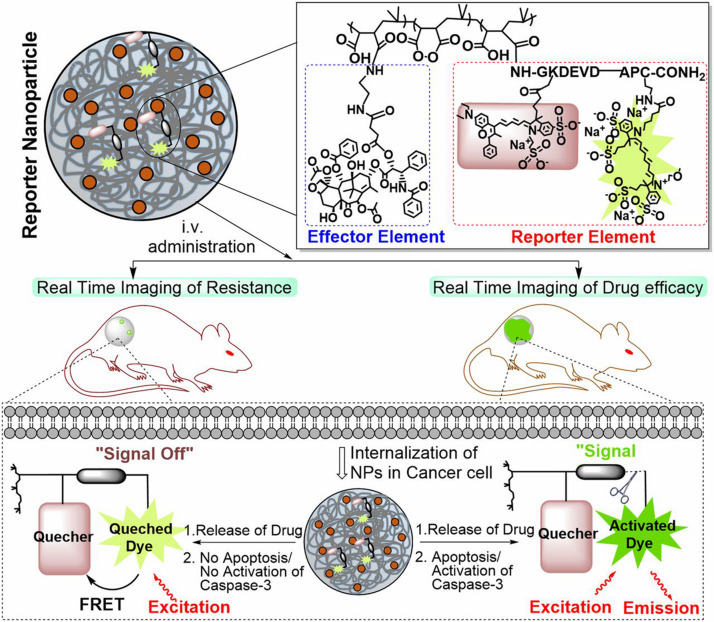
FIGURE 5.
Schematic illustration of the construct of a caspase-3 enzyme-responsive nanoparticle. The reporter nanoparticle comprises three components: a polymeric backbone, an esterase-cleavable prodrug synthesized from an anticancer drug [effector element (EE)], and an activatable reporter element (RE). At the optimal ratio of EE: RE, this stimuli-responsive polymer self-assembles into a nanoparticle. The reporter element is a caspase-3–cleavable sequence consisting of L-amino acids GKDEVDAPC-CONH2, to which we conjugated a FRET pair such that cleavage of the DEVD sequence results in removal of the quenching of the fluorescent signal. The effector element is conjugated to the polymeric backbone via an esterasecleavable bond, whereas the reporter element is conjugated via an amide bond with the Gly residue. In normal conditions, the fluorescent signal from the reporter element is in the off-state because the drug is intact inside the nanoparticle. In a drug-sensitive cell (lower right of the schematic), the released drug initiates apoptosis via the activation of the caspase-3 enzyme, which then cleaves the DEVD peptide, unquenching the fluorescent signal (on the state). However, in a non-responder cell (lower left), the failure of the released drug to induce apoptosis means the reporter element remains in the off state. Reprinted from Ashish Kulkarni, Poornima Rao, Siva Natarajan, et al. Reporter nanoparticle monitors its anticancer efficacy in real-time. Proc Natl Acad Sci U S A. 2016;113(15): E2104–13. With permission from reference (Kulkarni A. et al., 2016). FRET, Förster resonance energy transfer; DEVD, Asp-Glu-Val-Asp.